Properties, Preparation and Ionic Structure of Calcium Chloride
CaCl2, also known as Calcium Chloride, is an ionic compound commonly referred to as Calcium chloride anhydrous or Calcium dichloride. While discovered in the 15th century, it garnered little attention until the latter part of the 18th century. Initially, it was only studied with laboratory-prepared samples, as commercial production did not commence until after the ammonia-soda process for manufacturing soda ash was established.
Properties and Preparation
Calcium Chloride is an ionic compound composed of chlorine and calcium. At room temperature, it appears as a crystalline solid with a white color. Its high solubility in water renders it hygroscopic, odorless, and with a notably high enthalpy change of solution. Widely utilized for dust control and de-icing, it can be prepared through two methods:
1. Reacting calcium carbonate with hydrochloric acid.
2. Directly from limestone, with a significant portion also produced as a by-product of the Solvay process.
Ionic Structure
Calcium chloride molecules feature two ionic bonds between the single calcium cation and the two chloride anions. The structure of calcium chloride molecules is illustrated below.
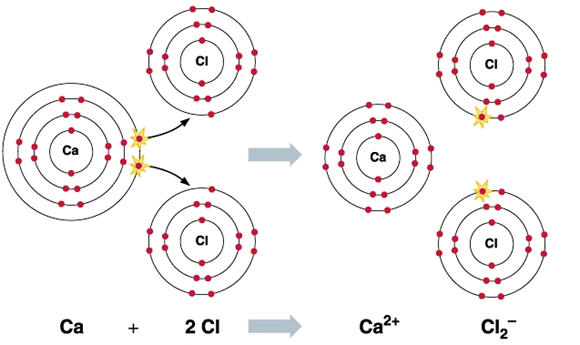
It can be noted that the calcium cation holds a charge of magnitude +2 and each chloride anion holds a charge of magnitude -1. The compound is, therefore, electrically neutral.
You may like
Related articles And Qustion
See also
Lastest Price from Calcium chloride manufacturers

US $1200.00-1100.00/ton2025-09-10
- CAS:
- 10043-52-4
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $0.00-0.00/KG2025-06-03
- CAS:
- 10043-52-4
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS