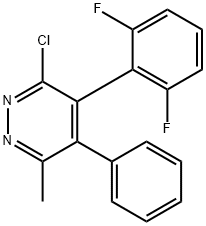
The brief introduction of Pyridochlometyl.
Overview
Pyridochlometyl was announced by Sumitomo Chemical Company as a new fungicide in 2017 and has been developed under the code S-2190. Pyridachlometyl exhibits potent fungicidal activity against a broad range of phytopathogens from the phyla of Ascomycota and Basidiomycota by promoting the tubulin polymerization (microtubule stabilization), which is an entirely novel mode of action for the fungicide market. Very similar tetrasubstituted pyridazines with the same substitution pattern of chloride and a methyl group next to both ring nitrogen atoms and two phenyl rings in between, one of them 2,6-difluorinated, have also been reported as disruptors of microtubule dynamics[1]. These closely related analogs of pyridochlometyl were found to possess strong fungicidal activity and have been tested against human African trypanosomiasis and Alzheimer's disease.
Pyridachlometyl exhibited potent antifungal activity against a broad range of fungal species belonging to the phyla Ascomycota and Basidiomycota. There was no cross-resistance with other fungicide classes, such as ergosterol biosynthesis inhibitors, respiratory inhibitors, or tubulin polymerization inhibitors in Zymoseptoria tritici. Pyridachlometyl-resistant strains were obtainable by UV mutagenesis in Z. tritici and Penicillium digitatum.
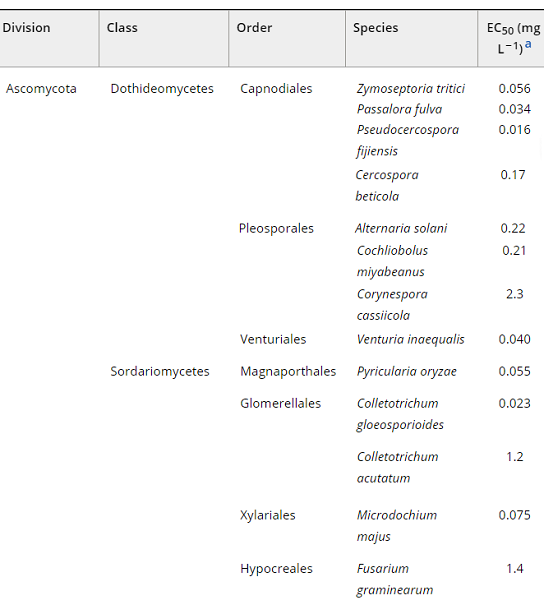
The antifungal spectrum of pyridachlometyl
As shown below, all fungal species tested were sensitive to pyridachlometyl (EC50 < 5 mg L−1). A correlation between pyridachlometyl's effectiveness and fungal classification was expected because of the similarity in the primary structure of tubulin proteins, but a relationship was not observed. For example, sensitivity significantly differed between two closely related species, such as Colletotrichum gloeosporioides and C. acutatum. However, the primary structures of their tubulin proteins, including the vital amino acids at putative binding sites, are almost identical[2]. This difference might be ascribed to the differential expression level of the gene encoding β-tubulin rather than the structural difference of tubulin proteins. In C. acutatum, the gene encoding β-tubulin was overexpressed in response to benomyl, a profungicide of carbendazim, and was mediated by a transcription factor-like protein containing a leucine-zipper motif. Furthermore, the tubulin gene copy numbers were different across species of fungi. For example, Fusarium graminearum, less sensitive to pyridachlometyl, is known to possess two divergent β-tubulin-encoding genes exhibiting 76.6% similarity in their putative amino acid sequences. Such redundancy of tubulin genes might also be related to the varying sensitivity of each species to pyridachlometyl.

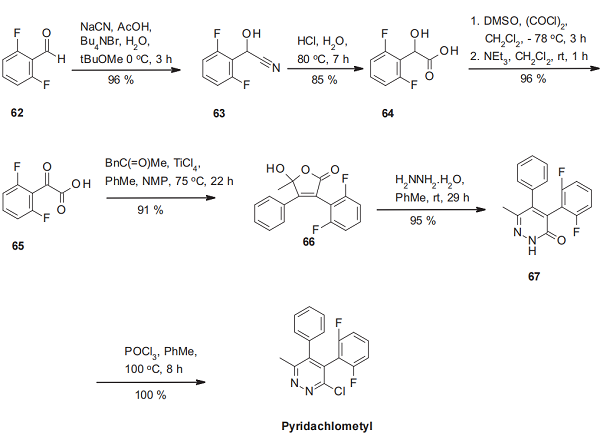
Synthesis method
The synthesis of pyridachlometyl starts with the transformation of 2,6-difluorobenzaldehyde (62) into its cyanohydrine derivative 63. Hydrolysis of the nitrile function delivers the difluorinated mandelic acid 64, converted into the corresponding phenyl glyoxylic acid 65. This α-ketoacid is directly reacted with methyl benzylmethyl ketone in the presence of titanium tetrachloride to yield the desired γ-hydroxybutenolide 66 by a Knoevenagel-type condensation. The ring enlargement of this hydroxyfuranone 66 with hydrazine hydrate to the pyridazinone 67 is then followed by the phosphorus oxychloride-mediated chlorination to pyridachlometyl.
References
[1] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.
[2] Yuichi Matsuzaki. “Pyridachlometyl has a novel anti-tubulin mode of action which could be useful in anti-resistance management.” Pest Management Science 76 4 (2019): 1393–1401.