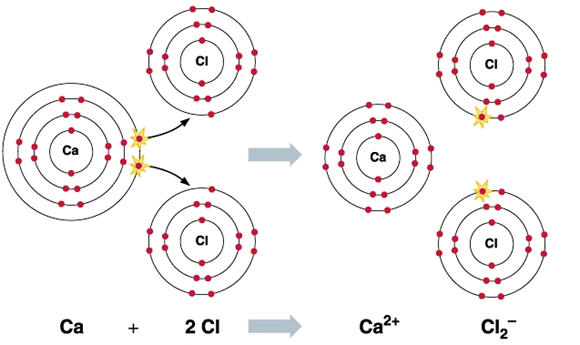
Synthesis of Calcium dichloride
General description
Calcium dichloride is a calcium salt and an inorganic chloride. It has a role as a fertilizer. Calcium chloride is a white to off-white solid. Sinks and mixes with water.Calcium chloride is an inorganic salt composed of chlorine and calcium. It has the characteristics of heating in water, low freezing point and strong water absorption. It has important application value in industry, food manufacturing, building materials, medicine and biology [1]. Main uses: first, it is widely used in road transportation industry, which is effectively used to melt ice and snow in expressways, urban roads and other places; Second, calcium chloride is easily hydrolyzed in the air, which can strongly adsorb the surrounding dust for mining and road dust removal in summer; Third, calcium chloride can be used as a component of drilling medium to help stabilize various mud layers at different depths, or as a component of drilling powder to play the role of drilling lubrication. It is used to remove SO42 -, desiccant, dehydrating agent, refrigerant, etc. in the industrial field; Fifth, in the food industry, it can be added to drinking water or beverages as seasoning and minerals, as coagulant in the production of bean products, or as calcium nutrient in food.
Application
Calcium dichloride used to remove SO42 -, desiccant, dehydrating agent, refrigerant, etc. in the industrial field; Fifth, in the food industry, it can be added to drinking water or beverages as seasoning and minerals, as coagulant in the production of bean products, or as calcium nutrient in food. It is used as a multi-purpose desiccant, such as nitrogen, oxygen, hydrogen, hydrogen chloride, sulfur dioxide and other gases. Used as dehydrating agent in the production of alcohol, ester, ether and acrylic resin. Calcium chloride aqueous solution is an important refrigerant for refrigerator and ice making. It can accelerate the hardening of concrete and increase the cold resistance of building mortar. It is an excellent building antifreeze. It can be used as fog eliminator, road dust collector and fabric fire retardant in port. Used as protective agent and refining agent for aluminum magnesium metallurgy. It is a precipitant for the production of lake pigments. Used for deinking waste paper processing. It is the raw material for the production of calcium salt.
Synthesis
According to different raw materials, domestic calcium chloride production processes are mainly divided into two categories, That is, the production process of making calcium carbonate solution by alkali (hereinafter referred to as "calcium by alkali") and the production process of dissolving calcium carbonate by hydrochloric acid (hereinafter referred to as "calcium by acid"). Calcium by alkali refers to the preparation of calcium chloride product by comprehensively recovering the calcium by alkali solution produced by ammonia alkali soda ash. Calcium by acid refers to the preparation of calcium chloride product by the reaction of calcium carbonate or limestone with hydrochloric acid.
Figure the systhesis route of Calcium dichloride
Firstly, the phosphorus ore is decomposed with hydrochloric acid to prepare a mixed solution containing Ca (mg) Cl2, H3PO4 and H2 SiF6. Then, the fluorine ion in the extraction solution is removed with limestone (precipitated in the form of CaF2 + SiO2), and then the phosphate in the defluorination solution is removed with limestone and lime milk (precipitated in the form of 2CaHPO4.H2O). Finally, a relatively pure Ca (mg) Cl2 solution is obtained. The precipitated CaF2 + SiO2 is filtered by a filter press to obtain fluorosilicone slag, which is added to the compound fertilizer as a soil conditioner; The precipitated 2cahpo4 · H2O is filtered by vacuum belt filter and then sent to the original calcium hydrogen phosphate drying device for drying. The by-product is high-quality calcium hydrogen phosphate (feed grade); The relatively pure Ca (mg) Cl2 solution is added with lime milk, the magnesium ions are precipitated in the form of Mg (OH) 2 precipitation, and then filtered by a filter press. The obtained demineralised slag is added to the compound fertilizer as a soil conditioner, and finally a more pure CaCl2 solution is obtained.[1]. The combination of "waste liquid recovery method + hydrochloric acid stone powder method" not only solves the outlet of by-product hydrochloric acid in some units, but also makes the production cost of calcium chloride relatively low. The production industrial chain introduced in this paper is set up reasonably, the raw materials - by-products - products, the trend of raw materials is reasonable, the comprehensive utilization of waste is realized, the full reuse of sewage after treatment is realized, there is no discharge, and there is no new harm to the environment. At the same time, it is greatly reduced on the basis of the original total discharge of "three wastes".[2].
Reference
1.Wang Xiaoyan: decomposition of phosphate rock by hydrochloric acid to produce calcium chloride, Shandong chemical industry, 2021, No. 02, pp. 117-118.
2.Liu Kai, song Xixi, Zhou Haifeng, etc.: calcium chloride products and their production process, China well mineral salt, 2020, No. 03, pp. 5-7.
You may like
Related articles And Qustion
Lastest Price from Calcium chloride manufacturers

US $1200.00-1100.00/ton2025-09-10
- CAS:
- 10043-52-4
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $0.00-0.00/KG2025-06-03
- CAS:
- 10043-52-4
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS