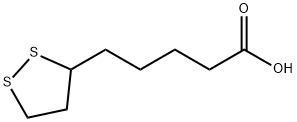
Systhesis of Lipoic acid
General description
α- Lipoic acid is light yellow powder crystal, almost tasteless Low solubility in water, about 1 g / L (20 ° C) Soluble in 10% NaOH solution and aliphatic solvent Soluble in methanol, ethanol, chloroform and ether Lipoic acid α- Lipoic acid Lipoic acid has a chiral center, so it has two configurations: R and s Natural lipoic acid is a white crystal. Only R-configuration and R-configuration lipoic acid can be covalently conjugated to lysine residues through amide bonds to play the function of coenzyme. (R)-lipoic acid is the (R)-enantiomer of lipoic acid. A vitamin-like, C8 thia fatty acid with anti-oxidant properties. It has a role as a prosthetic group, a nutraceutical and a cofactor. It is a lipoic acid, a member of dithiolanes, a heterocyclic fatty acid and a thia fatty acid. It derives from an octanoic acid. It is a conjugate acid of a (R)-lipoate. It is an enantiomer of a (S)-lipoic acid.
Application and pharmacology
Lipoic acid is an essential cofactor for mitochondrial metabolism and is synthesized denovo using intermediates from mitochondrial fatty acid synthesis type II, S-adenosylmethionine and iron-sulfur clusters. This cofactor is required for catalysis by multiple mitochondrial 2-ketoacid dehydrogenase complexes, including pyruvate dehydrogenase, alpha-ketoglutarate dehydrogenase, and branched-chain ketoacid dehydrogenase. Lipoic acid also plays a critical role in stabilizing and regulating these multi-enzyme complexes. Many of these dehydrogenases are regulated by reactive oxygen species, mediated through the disulfide bond of the prosthetic lipoyl moiety. Collectively, its functions explain why lipoic acid is required for cell growth, mitochondrial activity and coordination of fuel metabolism.
α-lipoic acid (LIPOIC ACID, thioctic acid) is an organosulfur component produced from plants, animals, and humans. It has various properties, among them great antioxidant potential and is widelyused as a racemic drug for diabetic polyneuropathy-associated pain and paresthesia. Naturally , LIPOIC ACID is located in mitochondria, where it is used as a cofactor for pyruvate dehydrogenase (PDH) andα-ketoglutarate dehydrogenase complexes. Although about- α- Important progress has been made in the study of asymmetric synthesis methods of lipoic acid, but most methods are still in the laboratory research stage. In general, these methods have one or more problems, such as long synthesis steps, poor enantioselectivity, low yield, harsh reaction conditions, the use of highly toxic reagents, expensive test reagents and so on. Therefore, the research and development of new methods, new technologies and new strategies to improve the overall synthesis efficiency is still the key to carry out synthesis (R) with industrial and practical value in the future- α- Key problems to be solved in the study of lipoic acid method. Organic small molecule catalysts have the advantages of low cost, easy structure regulation, good stability, metal free and even recyclable. However, in the reported methods, organic small molecule catalysts are used for asymmetric synthesis (R)- α. The method of lipoic acid has not been reported.
LIPOIC ACID has various benefits, including antioxidant potential; however, it has been shown that the therapeutic efficacy of LIPOIC ACID is restricted because of limitations related with its pharmacokinetic profile. Data shows a short half-life and bioavailability of about 30% of LIPOIC ACID due to mechanisms involving hepatic degradation, reduced LIPOIC ACID solubility as well as instability in the stomach. However, the use of various innovative formulations has proved to be effective in enhancing LIPOIC ACID bioavailability. It has been shown through studies that LIPOIC ACID bioavailability is enhanced through the use of amphiphilic matrices, able to enhance its solubility and absorption in the intestine. Moreover, LIPOIC ACID liquid formulations show greater plasma concentrations and bioavailability as compared to solid dosages. Moreover, age also affects LIPOIC ACID bioavailability , while gender shows insignificant differences. Thus, improved formulations that can enhance LIPOIC ACID absorption will markedly improve LIPOIC ACID bioavailability , ultimately leading to an improved therapeutic efficacy.[1,2]
Synthesis
In 2015, Xu et al. systematically studied the asymmetric reduction of ketones by using a reductase cpar2 isolated from c.parapsilosis. The reductase has high activity and chiral selectivity for the asymmetric reduction of ketone carbonyl in 8-chloro-6-carbonyloctanoate 15 (Fig. 1). Under the optimized conditions, the conversion of substrate is > 99%, the separation yield of chiral hydroxyl product 16A is 86%, and the E.E. value is > 99%. Starting from the chiral compound 16A as the key intermediate, the chiral hydroxyl group is chlorinated to form the configuration reversed dichlorocompound 17a, and then the target compound 1a is obtained by thio and ester hydrolysis.
Synthesis of ( R) -α-lipoic acid through the asymmetric reduction of β-ketone ester 15 by reductase
(R) - α- Lipoic acid is widely used, not only for the treatment of a variety of diseases, but also as health products. At present, the market demand for lipoic acid is huge, but due to its difficult synthesis, the supply is seriously insufficient and the market price is expensive. Therefore, it is very important to develop efficient synthesis methods with industrial application value. Although about- α- Important progress has been made in the study of asymmetric synthesis methods of lipoic acid, but most methods are still in the laboratory research stage. In general, these methods have one or more problems, such as long synthesis steps, poor enantioselectivity, low yield, harsh reaction conditions, the use of highly toxic reagents, expensive test reagents and so on. Therefore, the research and development of new methods, new technologies and new strategies to improve the overall synthesis efficiency is still the key to carry out synthesis (R) with industrial and practical value in the future- α- Key problems to be solved in the study of lipoic acid method. Organic small molecule catalysts have the advantages of low cost, easy structure regulation, good stability, metal free and even recyclable. However, in the reported methods, organic small molecule catalysts are used for asymmetric synthesis (R)- α The method of lipoic acid has not been reported.[3]
History
Lipoic acid (6,8-dithiooctanoic acid) was first identified by Lester Reed and colleagues in the 1950s (1-3), and over the last several decades much has been learned about the chemical properties and biological functions of this unique cofactor. The disulfide bond within the molecule provides a source of reductive potential that is required for catalysis by mitochondrial 2-ketoacid dehydrogenases, and participates in stabilization and redox dependent regulation of these multi-enzyme complexes. These functions make lipoic acid essential for cell growth, oxidation of carbohydrates, amino acids and other fuels, and regulating mitochondrial redox blipoic acidnce. Herein, we discuss the intricacies of lipoic acid synthesis in various organisms, the role lipoylation plays in 2-ketoacid dehydrogenase activities and regulation, and how these enzymes are influenced by reactive oxygen species through this covalently bound lipoic acid cofactor.
Reference
1.Salehi B., Berkay Yılmaz Y. & Antika G. et al., "Insights on the Use of α-Lipoic Acid for Therapeutic Purposes," Biomolecules, Vol.9, No.8(2019), p.356.
2.Solmonson A. & DeBerardinis R. J., "Lipoic acid metabolism and mitochondrial redox regulation," Journal of Biological Chemistry, Vol.293, No.20(2018), pp.7522-7530.
3.Han Xiu, Kang Chuanqing: (R)- α- Research Progress on synthesis methods of lipoic acid, applied chemistry, No. 11, 2020, pp. 1236-1248.
Reference
1.Salehi B., Berkay Y?lmaz Y. & Antika G. et al., "Insights on the Use of α-Lipoic Acid for Therapeutic Purposes," Biomolecules, Vol.9, No.8(2019), p.356.
2.Solmonson A. & DeBerardinis R. J., "Lipoic acid metabolism and mitochondrial redox regulation," Journal of Biological Chemistry, Vol.293, No.20(2018), pp.7522-7530.
3.Han Xiu, Kang Chuanqing: (R)- α- Research Progress on synthesis methods of lipoic acid, applied chemistry, No. 11, 2020, pp. 1236-1248.
You may like
Related articles And Qustion
Lastest Price from α-Lipoic Acid manufacturers

US $1.00-100.00/KG2025-09-04
- CAS:
- 1077-28-7
- Min. Order:
- 1KG
- Purity:
- 95%
- Supply Ability:
- 300KG

US $0.00-0.00/kg2025-08-19
- CAS:
- 1077-28-7
- Min. Order:
- 1kg
- Purity:
- 99%-101%; USP
- Supply Ability:
- 1000T