Preparation and Flame Test of Calcium Chloride
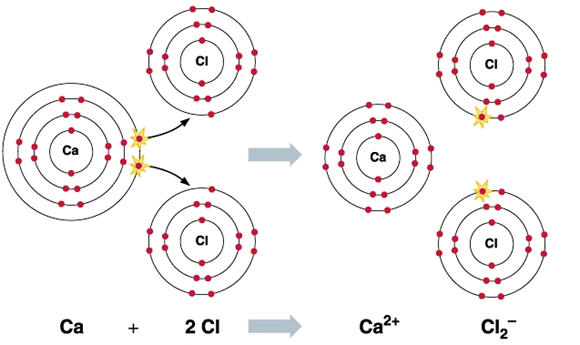
CaCl2 is an ionic compound with chemical name Calcium Chloride. It is also called Calcium chloride anhydrous or Calcium dichloride. It is an ionic compound of chlorine and calcium. At room temperature, it is a crystalline solid white in colour. It is highly soluble in water and hence is hygroscopic in nature. It is odourless and has a very high enthalpy change of solution. This compound is widely used for dust control and de-icing.
Preparation of Calcium Chloride
The steps listed below can be followed in order to prepare calcium chloride:
Step 1: Take a beaker. Wear gloves and place limestones in it until the beaker is filled up by a quarter of its total volume.
Step 2: Add approximately 1/4th of a beaker of HCl (hydrochloric acid) to the limestones.
Step 3: As the HCl dissolves the limestone it starts to bubble. Mix the
contents in the beaker gently and take care that the reaction completes.
Add a little limestone if all the limestones dissolve in it completely.
Step 4: Filter off the solids by pouring the solution through the filter paper as soon as the solution stops bubbling.
Step 5: Heat the second beaker which contains the calcium chloride solution.
Solid calcium chloride is the solid left after the water evaporates.
Flame Test of Calcium Chloride

The picture above is the flame test of calcium chloride. A flame test is a complex phenomenon that is not fully explained. In simple words, when a solution of metal salts, e.g., an aqueous solution of metal chlorides is injected into a flame, some of the metal ions may gain electrons and become neutral metal atoms. Electrons in the atom can be promoted from the ground state to a higher energy excited state by the strong heat of the flame. The excited electrons ultimately return to the ground state either in one go or in several steps by jumping to lower allowed energy states. When the excited electrons jump from higher to lower allowed energy states they emit electromagnetic radiation of a specific wavelength corresponding to the energy gap between the energy states. Some of these radiations may fall in the visible part of the electromagnetic radiation spectrum. Therefore, when we burn calcium chloride, the flame will appear orange-red.
Related articles And Qustion
Lastest Price from Calcium chloride manufacturers

US $1200.00-1100.00/ton2025-09-10
- CAS:
- 10043-52-4
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $0.00-0.00/KG2025-06-03
- CAS:
- 10043-52-4
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS