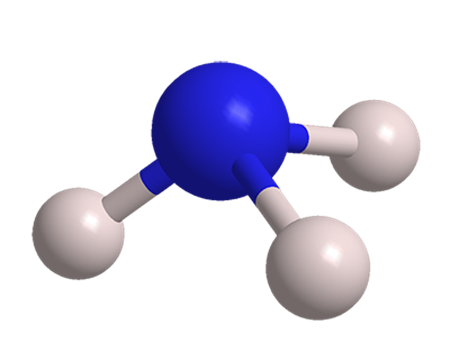
What is the formula and change of Ammonia?
Ammonia is an inorganic chemical compound with the formula NH3.

Ammonia is the simplest stable compound of these elements and serves as a starting material for the production of many commercially important nitrogen compounds.
Properties
Ammonia is a colorless gas with a characteristically pungent smell. It is lighter than air, its density being 0.589 times that of air. It is easily liquefied due to the strong hydrogen bonding between molecules. Gaseous ammonia turns to a colorless liquid, which boils at −33.1 °C (−27.58 °F) and freezes to colorless crystals at −77.7 °C (−107.86 °F). Little data is available at very high temperatures and pressures, such as supercritical conditions.
Ammonia change
Like water, liquid ammonia undergoes molecular autoionization to form its acid and base conjugates:
2 NH3 ⇌ NH+4 + NH−2
Ammonia often functions as a weak base, so it has some buffering ability. Shifts in pH will cause more or fewer ammonium cations (NH+4) and amide anions (NH−2) to be present in solution. At standard pressure and temperature,
K = [NH+4] × [NH−2] = 10−30.
Toxicity
The toxicity of ammonia solutions does not usually cause problems for humans and other mammals, as a specific mechanism exists to prevent its build-up in the bloodstream. Ammonia is converted to carbamoyl phosphate by the enzyme carbamoyl phosphate synthetase and then enters the urea cycle to be either incorporated into amino acids or excreted in the urine.
Fish and amphibians lack this mechanism, as they can usually eliminate ammonia from their bodies by direct excretion. Ammonia even at dilute concentrations is highly toxic to aquatic animals, and for this reason, it is classified as dangerous for the environment. Atmospheric ammonia plays a key role in the formation of fine particulate matter.
You may like
Related articles And Qustion
Lastest Price from Ammonia manufacturers

US $2.00/kg2024-12-17
- CAS:
- 7664-41-7
- Min. Order:
- 10000kg
- Purity:
- 99%
- Supply Ability:
- 10000000