Candesartan cilexetil: Pharmacodynamics, Pharmacokinetics and Therapeutic Uses
General Description
Candesartan cilexetil is an angiotensin II receptor antagonist used for treating hypertension. Its insurmountable binding to the angiotensin II receptor subtype 1 blocks its activation, leading to reduced blood pressure and improved cardiac function. The drug's pharmacokinetics include rapid absorption, high plasma protein binding, and minor metabolism. Clinical studies have demonstrated dose-dependent reductions in blood pressure, with comparable efficacy to other antihypertensive agents. Notably, candesartan cilexetil has shown benefits in patients with renal failure, type 2 diabetes mellitus, and microalbuminuria. Overall, candesartan cilexetil is a valuable option for managing hypertension, offering reliable and sustained blood pressure control.

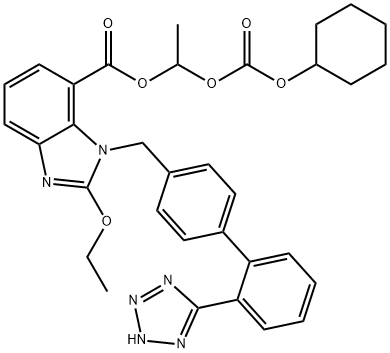
Figure 1. Candesartan cilexetil
Pharmacodynamics
Candesartan cilexetil, a medication used in the treatment of hypertension, exerts its pharmacodynamic properties through selective and insurmountable binding to the angiotensin II receptor subtype 1 (AT1). By inhibiting the binding of angiotensin II, candesartan effectively blocks the AT1 receptors, a mechanism more potent than other similar drugs in vitro. Notably, candesartan has no impact on the angiotensin II receptor subtype 2 (AT2). In clinical studies, candesartan cilexetil demonstrated a dose-dependent attenuation of the pressor response to exogenous angiotensin II. It was observed that a significantly higher amount of angiotensin II was required to achieve the same increase in diastolic blood pressure (DBP) following treatment with therapeutic doses of candesartan cilexetil in healthy individuals. Furthermore, research has shown that candesartan cilexetil reduces left ventricular mass in hypertensive patients when administered at doses ranging from 2 to 16 mg/day. Additionally, at doses of 8 or 16 mg/day, candesartan cilexetil was found to decrease renal vascular resistance and reduce urinary albumin and protein excretion in patients with hypertension and coexisting type 2 diabetes mellitus and microalbuminuria or proteinuria. Moreover, treatment with candesartan cilexetil at 8 or 16 mg/day restored impaired tonic nitric oxide release in hypertensive patients. The drug's effect on nitric oxide release may be linked to its mechanism of action or attributed to the significant reductions in blood pressure. Interestingly, the vasodilatory response to TAK-044, an endothelin A/B receptor antagonist, was notably reduced in these patients receiving candesartan cilexetil. 1
The effect of candesartan cilexetil on neurohormone levels was assessed in two large, randomised, double-blind, multicentre trials in patients with CHF (all or almost all of whom had New York Heart Association [NYHA] class ll or Il CHF at baseline).
The smaller, 3-month trial (n= 218) compared candesartan cilexetil 2, 4, 8 or 16 mg once daily with placebo; a 2-week placebo run-in period preceded randomisation. Some background CHF therapies were allowed during the trial; those not permitted included ACE inhibitors, angiotensin II-receptor an tagonists (apart from the study drug) and renin antagonists.
Pharmacokinetics
After oral administration, candesartan cilexetil undergoes absorption in the gastrointestinal tract and undergoes rapid and complete metabolism to its active form, candesartan. Peak plasma concentrations of candesartan are typically achieved within about 3 to 5 hours following the administration of candesartan cilexetil at doses ranging from 4 to 16 mg, with a dose-dependent increase in concentration. There is no significant drug accumulation observed with once-daily dosing. The absolute oral bioavailability of candesartan is approximately 15% and is not influenced by food intake. The drug exhibits high plasma protein binding (>99%) and has a relatively small volume of distribution (0.13 L/kg) following intravenous administration. Candesartan is predominantly excreted unchanged, with only a minor portion being metabolized by cytochrome P450 (CYP) 2C9 to the inactive metabolite CV-15959. The clearance rate of an intravenous dose of candesartan 4 mg is approximately 0.022 L/h/kg in healthy individuals, while oral clearance in hypertensive patients receiving candesartan cilexetil 2 to 16 mg/day is around 14.07 L/h. Following oral administration of a labeled dose of candesartan cilexetil, approximately 68% of radioactivity is recovered in feces and 33% in urine, with at least 90% eliminated within 72 hours. The elimination half-life of candesartan ranges from approximately 9 to 13 hours and is not dose-dependent. 2
Therapeutic Uses
The clinical efficacy of candesartan cilexetil in adult patients with heart failure from ischaemic and/or non-ischaemic causes was assessed in several randomised, double-blind, multicentre trials.
These included the pivotal CHARM (Candesartan in Heart failure - Assessment of Reduction in Mortality and morbidity) programme, which comprised three separate component trials in a total of 7599 patients. A prespecified analysis of CHARM participants with LVEF <40% was subsequently undertaken. Several earlier trials are also reviewed in this section; however, discussion focuses on the CHARM programme.
In addition to the inclusion criteria, patients in CHARM-Preserved and those with NYHA class I CHF in CHARM-Added were required to have been hospitalised for a cardiac reason at any time or in the previous 6 months. Over two-thirds of patients in CHARM-Alternative had also previously been hospitalised for CHF.1
Reference
1. Easthope SE, Jarvis B. Candesartan cilexetil: an update of its use in essential hypertension. Drugs. 2002;62(8):1253-1287.
2. de Zeeuw D, Remuzzi G, Kirch W. Pharmacokinetics of candesartan cilexetil in patients with renal or hepatic impairment. J Hum Hypertens. 1997;11 Suppl 2:S37-S42.
Related articles And Qustion
Lastest Price from Candesartan cilexetil manufacturers

US $5.00-0.50/KG2025-05-30
- CAS:
- 145040-37-5
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $5.00-0.50/KG2025-05-08
- CAS:
- 145040-37-5
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS