Ammonia synthesis and Ammonia uses
Ammonia is a colorless highly irritating gas with a sharp suffocating odor. It dissolves easily in water to form ammonium hydroxide solution which can cause irritation and burns. Ammonia gas is easily compressed and forms a clear, colorless liquid under pressure. It is usually shipped as a compressed liquid in steel cylinders. Ammonia is not highly flammable, but containers of ammonia may explode when exposed to high heat.

Synthesis
For small scale laboratory synthesis, one can heat urea and calcium hydroxide or sodium hydroxide:
(NH2)2CO + Ca(OH)2 → CaCO3 + 2 NH3
Ammonia is synthesized industrially through the Haber process, it is the main industrial procedure for the production of ammonia. It converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using an iron metal catalyst:
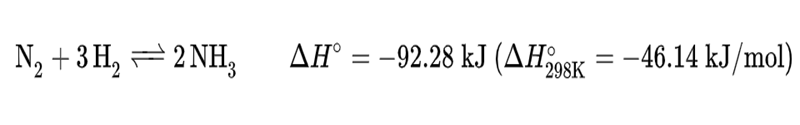
This reaction is slightly favorable in terms of enthalpy, but is disfavored in terms of entropy because four equivalents of reactant gases are converted into two equivalents of product gas. As a result, high pressures and moderately high temperatures are needed to drive the reaction forward.
Uses
About 80% of the ammonia produced in industry is used in agriculture as fertilizer. Ammonia is also used as a refrigerant gas, to purify water supplies, and in the manufacture of plastics, explosives, fabrics, pesticides, dyes and other chemicals. It is found in many household and industrial-strength cleaning solutions. Cleaning solutions for industrial use contain higher concentrations of ammonia and can quickly cause irritation and burns.
Toxicity
The toxicity of ammonia solutions does not usually cause problems for humans and other mammals, as a specific mechanism exists to prevent its build-up in the bloodstream. Ammonia is converted to carbamoyl phosphate by the enzyme carbamoyl phosphate synthetase, and then enters the urea cycle to be either incorporated into amino acids or excreted in the urine.[131] Fish and amphibians lack this mechanism, as they can usually eliminate ammonia from their bodies by direct excretion. Ammonia even at dilute concentrations is highly toxic to aquatic animals, and for this reason it is classified as dangerous for the environment. Atmospheric ammonia plays a key role in the formation of fine particulate matter.
You may like
Related articles And Qustion
See also
Lastest Price from Ammonia manufacturers

US $2.00/kg2024-12-17
- CAS:
- 7664-41-7
- Min. Order:
- 10000kg
- Purity:
- 99%
- Supply Ability:
- 10000000