What is the crystal structure of Zirconium dioxide?
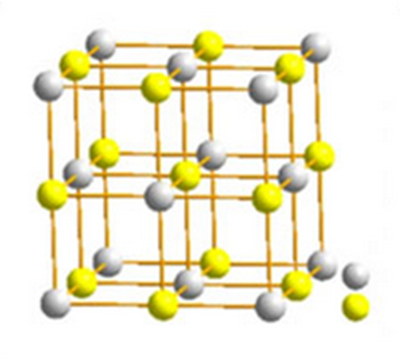
Zirconium dioxide (ZrO2), also known as zirconia and zirconium oxide, is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the rare mineral, baddeleyite. It is characterised by its high thermal resistivity, mechanical resistance, and abrasive properties.
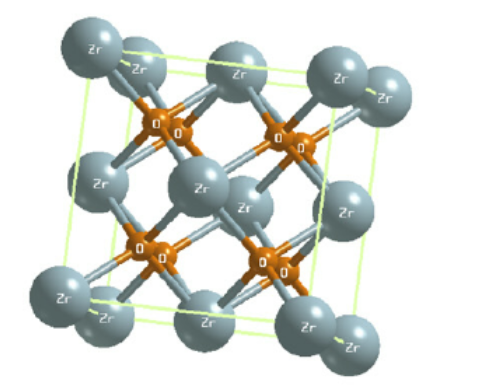
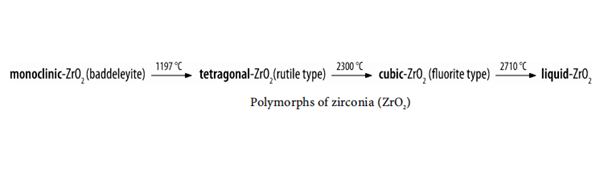
Zirconium dioxide adopts a monoclinic crystal structure at room temperature and transitions to tetragonal and cubic at higher temperatures. The change of volume caused by the structure transitions from tetragonal to monoclinic to cubic induces large stresses, causing it to crack upon cooling from high temperatures.
The cubic phase of zirconia also has a very low thermal conductivity, which has led to its use as a thermal barrier coating or TBC in jet turbine and diesel engines to allow operation at higher temperatures. Stabilized zirconia is used in oxygen sensors and fuel cell membranes because it has the ability to allow oxygen ions to move freely through the crystal structure at high temperatures. This high ionic conductivity (and a low electronic conductivity) makes it one of the most useful electroceramics.
You may like
Related articles And Qustion
Lastest Price from Zirconium dioxide manufacturers

US $0.00-0.00/kg2025-05-23
- CAS:
- 1314-23-4
- Min. Order:
- 1kg
- Purity:
- 99.7%min
- Supply Ability:
- 20mt

US $1.00/PCS2025-04-21
- CAS:
- 1314-23-4
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 100mt