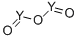What is the crystal structure of Yttrium oxide?
Yttrium oxide, with the chemical formula Y2O3, is known to exhibit different crystal structures under various conditions. The most common and stable form of yttrium oxide is the cubic phase, often referred to as C-type or C-Y2O3. This phase is characterized by a body-centered cubic (BCC) structure, also known as bixbyite or cubic-C structure.
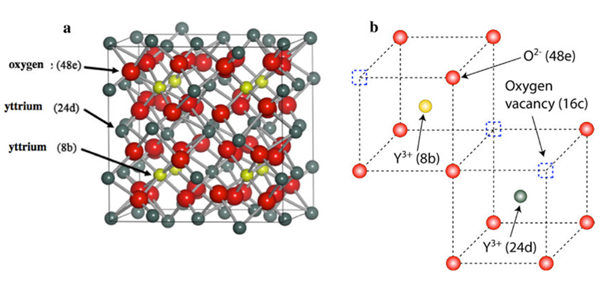
Structure of the cubic C-type Y2O3 (bixbyite). The lattice parameter is a 0 = 1.0604 nm. (a) Unit cell representation. (b) Environment of the two Y sites (the actual position of the O atoms is slightly shifted from the corners of the cubes). The small squares are structural oxygen vacancies.
The unit cell of the Y2O3cubic-Cstructure is composed of 80 atoms with a bulk lattice constant of a0=1.0604 nm.The oxygen atoms arelocated on the (48e) sites, and yttrium atoms are located on two non-equivalent cation sites, Y1(8b) and Y2(24d)(Fig. 1-a). All of the cations are six-fold coordinated: the Y1atoms aresurrounded by six neighbouring oxygens at the same distance(0.230 nm), whereas the Y2atoms have three pairs of neighbouring ox-ygens at three different distances (0.225, 0.228, and 0.236 nm). Thisstructure results in a specific arrangement of structural oxygen vacan-cies that form a network in the (16c) position along the body diagonaland face diagonal of the cubic cell(Fig. 1-b).
References:
[1] R.J. GABORIAUD; B. L; F Paumier. Disorder–order phase transformation in a fluorite-related oxide thin film: In-situ X-ray diffraction and modelling of the residual stress effects[J]. Thin Solid Films, 2016. DOI:10.1016/j.tsf.2015.08.030.
Related articles And Qustion
See also
Lastest Price from Yttrium oxide manufacturers

US $1.00-4.00/KG2025-09-01
- CAS:
- 1314-36-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $1.00-2.10/KG2025-08-29
- CAS:
- 1314-36-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20000KG