What is Tegoprazan?
Tegoprazan was approved by the Ministry of Food and Drug Safety (MFDS) for marketing in July 2018 for the treatment of gastroesophageal reflux disease and erosive esophagitis. Tegoprazan was originally developed by Pfizer. In 2008, it was licensed to RaQualiaPharma (separated from Pfizer) for joint development. In 2014, Tegoprazan was licensed to CJHealthCare by RaQualiaPharma. Finally, CJHealthCare was successfully developed and marketed in Korea. Tegoprazan is a competitive potassium ion acid blocker (P-CAB) and hydrogen ion/potassium ion exchange ATPase (H+/K+ATPase) inhibitor. The drug was first marketed in South Korea. Medicines for treating gastroesophageal reflux disease and erosive esophagitis. Proton pump hydrogen ion/potassium ion exchange ATPase is the main pharmacological target for the treatment of gastric acid-related diseases. Potassium-competitive acid blocker (P-CAB) can inhibit gastric acid secretion by competitively binding to K+ with H+/K+-ATPase. Research finds that Tegoprazan is such a potassium-competitive acid blocker and is considered to be the most advanced drug for treating gastroesophageal reflux disease, because proton pump inhibitors are the most commonly used drugs for treating gastroesophageal reflux disease. Tegoprazan The shortcomings of proton pump inhibitors can be just overcome. Tegoprazan's effectiveness and safety are mainly based on two phase III clinical trials. One of them is a double-blind, active-controlled phase III study. This study was conducted in South Korea. The study used 280 patients with erosive esophagitis as the primary endpoint[1].
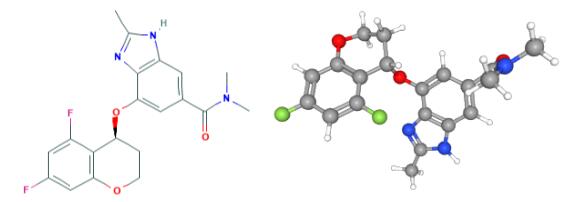
Fig 1. Chemical structure formula and three-dimensional structure of Tegoprazan
Tegoprazan, a potassium-competitive acid blocker, is a potent, oral active and highly selective inhibitor of gastric H+/K+-ATPase that could control gastric acid secretion and motility, with IC50 values ranging from 0.29-0.52 μM for porcine, canine, and human H+/K+-ATPases in vitro.
Tegoprazan inhibits porcine, canine, and human H+/K+-ATPase activity. Tegoprazan inhibits gastric H+/K+-ATPase in a potassium-competitive and reversible manner. Tegoprazan (3 μM) inhibits 86% of H+/K+-ATPase activity, whereas the inhibition is decreased to 34% after the dilution of Tegoprazan concentration to 0.15 μM[2].
Tegoprazan (1.0 mg/kg, p.o.) potently and completely inhibits histamine-induced gastric acid secretion in dogs. Tegoprazan (1.0-3.0 mg/kg, p.o.) reverses the pentagastrin-induced acidified gastric pH to the neutral range. Tegoprazan (3 mg/kg, p.o.) immediately evokes a gastric phase III contraction of the migrating motor complex in pentagastrin-treated dogs[3].
The invention relates to a method for preparing Tegoprazan chiral alcohols, in particular to the preparation method of (S) 5,7 difluoro 3,4 dihydro 2H chromogenic ene 4 alcohol. Using 5,7-difluoro-4H-benzopyran-4-ketone as starting material, the method realizes the preparation of (S)5,7-difluoro-3,4-dihydro-2H-chromogenic enone-4-alcohol by asymmetric reduction of ketone carbonyl with chiral reagent and subsequent conventional hydrogenation reaction[4].
References
[1] Takahashi N, et al. Tegoprazan, a Novel Potassium-Competitive Acid Blocker to Control Gastric Acid Secretion and Motility. J Pharmacol Exp Ther. 2018 Feb;364(2):275-286.
[2] Nobuyuki Takahashi and Yukinori Take.Journal of Pharmacology and Experimental Therapeutics February 2018, 364 (2) 275-286.
[3] Kim HK, Park SH, Cheung DY, Cho YS, Kim JI, Kim SS, Chae HS, Kim JK, and Chung IS (2010) Clinical trial: inhibitory effect of revaprazan on gastric acid secretion in healthy male subjects. J Gastroenterol Hepatol 25:1618–1625.
Mikami T, Ochi Y, Suzuki K, Saito T, Sugie Y, and Sakakibara M (2008) 5-Amino-6-chloro-N-[(1-isobutylpiperidin-4-yl)methyl]-2-methylimidazo[1,2-α]pyridine-8-carboxamide (CJ-033,466), a novel and selective 5-hydroxytryptamine4 receptor partial agonist: pharmacological profile in vitro and gastroprokinetic effect in conscious dogs. J Pharmacol Exp Ther 325:190–199.
You may like
Related articles And Qustion
See also

US $2.00-6.00/kg2025-07-29
- CAS:
- 942195-55-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg
US $0.00-0.00/g2025-04-20
- CAS:
- 942195-55-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 10000
![127852-28-2 (R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanol;Application; Use](/NewsImg/2023-12-25/6383911741532412169720831.jpg)
![127852-28-2 (R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanol;Application; Use](httpss://www.chemicalbook.com/NewsImg/2020-2-24/20202241719318661.jpg)
