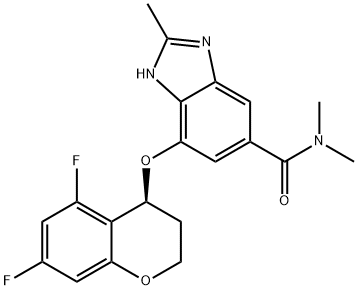
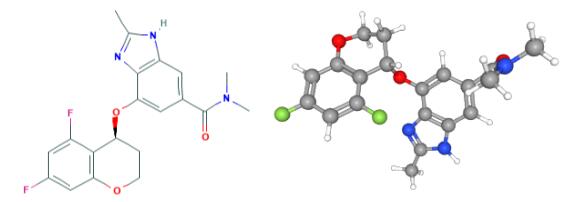
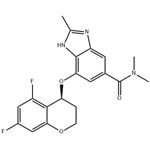
How to synthesize Tegoprazan?
Description
Tegoprazan is a novel orally active potassium-competitive acid blocker (P-CAB). It binds to H+/K+-ATPase competitively and reversibly (hydrogen bonding and electrostatic interactions). P-CABs do not require activation by acid; therefore, they are effective in environments with high intracellular pH. P-CABs can simultaneously inhibit resting and activated H+/K+-ATPase, resulting in a faster onset of action and longer-lasting acid inhibition than PPIs. Tegoprazan was approved for marketing in 2018 in South Korea for treating oesophagitis and gastroesophageal reflux disease and became available in China for oesophagitis in 2022. Several clinical trials have demonstrated the efficacy and tolerability of tegoprazan in treating other ARDs, such as Helicobacter pylori eradication[1].
In phase III clinical trials, once-daily dosing of the drug (50 mg or 100 mg) to patients with erosive esophagitis demonstrated noninferiority to esomeprazole (40 mg per day) in both healing and tolerability, supporting its approval in Korea in 2018. Tegoprazan, therefore, represents an alternative treatment approach to proton pump inhibitors (PPIs) such as esomeprazole, lansoprazole, omeprazole, and rabeprazole.
Synthetic method
The most likely scalable synthesis of tegoprazan has been described in an original patent filed by RaQualia[2]. Key challenges in the drug preparation are the construction of the tetrasubstituted aryl core and the efficient introduction of the enantiomerically pure chromanol side chain.
Preparation of Tegoprazan Chromanol
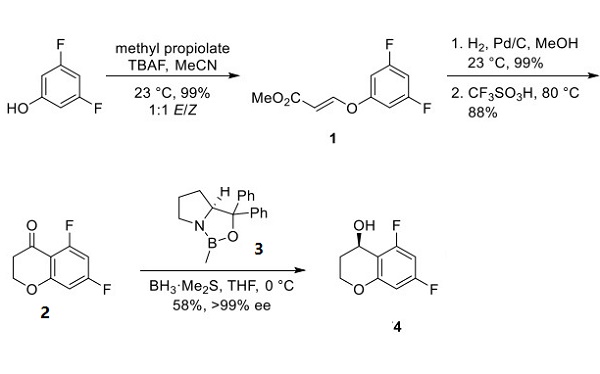
Chromanol 4 was prepared by the condensation of 3,5-difluorophenol with methyl propiolate using tetrabutylammonium fluoride (TBAF) as a base, affording 1 as a 1:1 mixture of E and Z enol ethers. Reduction of the double bond using catalytic hydrogenation and treatment with triflic acid facilitated an intramolecular Friedel−Crafts acylation to yield chromanone 2 in good yield for each step. Asymmetric reduction of ketone 2 with oxazaborolidine catalyst 3 using borane−dimethyl sulfide as the stoichiometric reductant provided alcohol 4 in 88% yield and 86% ee, which could be further resolved to >99% ee upon recrystallization.
Synthesis of Tegoprazan
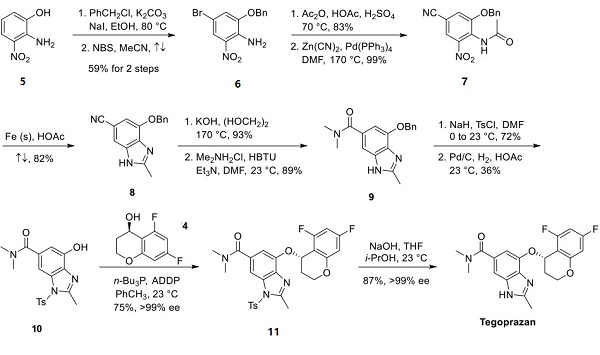
Starting with phenol 5, deprotonation of the phenol led to enhanced nucleophilicity, enabling selective Obenzylation. Bromination with NBS para to the aniline functionality afforded compound 6, an intermediate containing four appropriately placed functional groups in the heterocyclic core. Acetylation of aniline 6 generated a precursor to the methyl benzimidazole, which was then subjected to palladium-catalyzed cyanation of the aryl bromide at high temperature under microwave irradiation to yield compound 7. Iron-catalyzed reduction of the nitro group and concomitant condensation with the proximal N-acetyl functionality secured benzimidazole 8. Hydrolysis of the nitrile required forcing conditions, most likely due to the benzimidazole N−H functionality. Coupling of the resulting carboxylic acid with dimethylamine hydrochloride afforded amide 9. To make way for chromanol side chain addition, benzimidazole 9 was first protected as the tosylate prior to benzyl group removal via hydrogenolysis to give tosyl benzimidazole 10. Treatment of compound 10 and enantiomerically pure alcohol 4 in tri-n-butylphosphine with 1,1′-(azodicarbonyl)dipiperidine (ADDP) followed by silica gel purification and recrystallization afforded ether 11 in high enantiomeric excess and good yield. Removal of the tosyl group under basic conditions provided tegoprazan in 87% yield (>99% ee).
References
[1] Kaijing Guo . “Characterisation of degradation products of tegoprazan by LC-MS and GC-MS.” Journal of pharmaceutical and biomedical analysis 228 (2023): Article 115323.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
You may like
Related articles And Qustion
See also

US $2.00-6.00/kg2025-07-29
- CAS:
- 942195-55-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg
US $0.00-0.00/g2025-04-20
- CAS:
- 942195-55-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 10000