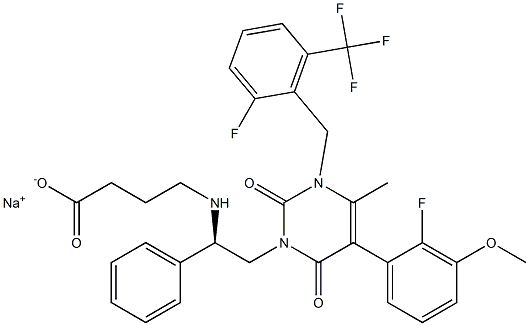
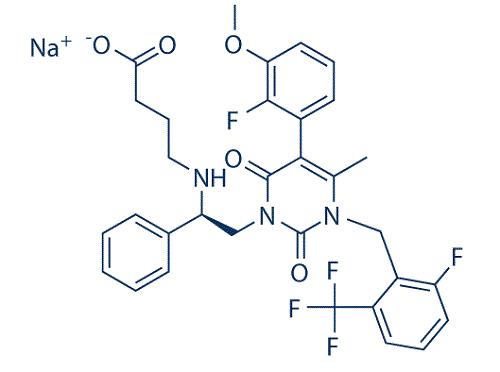
A non-peptide GnRH receptor antagonist: Elagolix
Description
Elagolix (ORILISSA™) is a small molecule, second-generation, non-peptide GnRH receptor antagonist that reduces levels of ovarian sex hormones in the blood. It was developed by AbbVie and Neurocrine Biosciences and approved by the USFDA in July 2018 for the treatment of women with endometriosis, a chronic disease resulting in intermenstrual bleeding, nonmenstrual pelvic pain, and pain during menstruation, intercourse, urination, and defecation.
Mechanism of action
Elagolix is a highly potent (KD = 54 pM) GnRH receptor antagonist that inhibits endogenous GnRH signaling by binding competitively to GnRH receptors in the anterior pituitary gland. Elagolix mechanism of action (MoA) differs from long-acting GnRH receptor agonists, which induce 1–2 weeks of 'flare-up' by downregulating GnRH receptors. The competitive nature of elagolix competitive antagonism of the GnRH receptors provides an advantage by allowing for rapid and reversible onset and offset, and hence more flexibility in modulating the hypothalamic–pituitary–gonadal axis[1].
It is active by suppression of luteinizing hormone and follicle-stimulating hormone. Because GnRH antagonists reduce estrogen release, these drugs are less prone to side effects related to complete estrogen suppression, such as bone mineral density loss. Based on the outcome of the phase III clinic trial, women who were treated with elagolix showed improved productivity at work, less absenteeism, and a significant decrease in reported levels of fatigue.
Synthetic method

Neurocrine Biosciences, Inc. has published multiple synthetic routes to elagolix, with both a milligram and multikilogram scale demonstration[2]. The synthetic route shown above represents the most likely process scale route, beginning with the condensation of urea and tertbutyl acetoacetate (1) under acidic conditions to generate uracil 2. Iodination of the uracil using iodine monochloride provided 3 in 90% yield, followed by a Suzuki coupling with aryl boronic acid 4. These conditions provided 5 high yields and were demonstrated on a multikilogram scale. Alkylation of uracil 5 with mesylate 7 (formed by the reaction of (−)-N-Boc-D-α-phenylglycinol with methane sulfonyl chloride (6)) followed by an acidic workup gave rise to the polysubstituted uracil core 8. Alkylation of the primary amine with ethyl 4-bromobutyrate enabled access to ester 9, which required filtration through a plug of silica gel for purification. Treatment of the ethyl ester with ethanolic sodium hydroxide followed by heptane recrystallization provided the target compound elagolix sodium in 78% yield.
References
[1] Mohamad Shebley. “Clinical Pharmacology of Elagolix: An Oral Gonadotropin-Releasing Hormone Receptor Antagonist for Endometriosis.” Clinical Pharmacokinetics 59 1 (2020): 297–309.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
You may like
Related articles And Qustion
Lastest Price from Elagolix Sodium manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 832720-36-2
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $10.00/KG2025-04-21
- CAS:
- 832720-36-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt