Description
Elagolix sodium (ABT-620) is a small molecule, orally active gonadotropin-releasing hormone (GnRH) receptor antagonist. It has the ability to suppress estradiol (E2) concentrations in a dose-dependent manner.
It was originally developed by Neurocrine Biosciences, then licensed to Abbott (now Abbvie) for worldwide co-development of this indication in 2010. AbbVie is applying to use elagolix for the treatment of endometriosis with associated pain. Elagolix is the first orally available GnRH antagonist, and as such may provide patients with relief from the painful symptoms of endometriosis while offering advantages over currently available injectable peptide-based treatments. The estimated patient population is over 170 million women worldwide.
Description
Elagolix is an antagonist of the gonadotropin-releasing hormone receptor (GnRHR; K
i = 0.9 nM in a radioligand binding assay). It is selective for GnRHR over the cytochrome P450 (CYP) isoform CYP3A4 (IC
50 = 56 μM) as well as a panel of 100 receptors, ion channels, enzymes, and transporters (IC
50s = >10 μM). Elagolix inhibits GnRH-induced inositol phosphate production in RBL-1 cells expressing human GnRHR (IC
50 = 1.5 nM).
In vivo, elagolix (30 mg/kg) suppresses production of luteinizing hormone in castrated male cynomolgus macaques. Formulations containing elagolix have been used in the treatment of endometriosis.
Chemical Properties
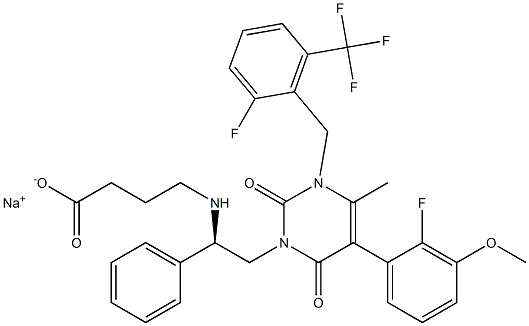
Elagolix sodium is a small synthetic molecule chemically known as Sodium 4-({(1R)-2- [5-(2-fluoro-3-methoxyphenyl)-3-{[2-fluoro-6-(trifluoromethyl) phenyl]methyl}-4- methyl -2,6-dioxo-3,6-dihydropyrimidin-1(2H)-yl]-1-phenylethyl} amino)butanoate. The structure was confirmed by mass spectrometry, infrared spectroscopy, nuclear magnetic resonance.
Elagolix sodium is isolated as a hygroscopic, amorphous white to off-white to light yellow powder.
Uses
Elagolix Sodium is a novel uracil phenylethylamine that acts as potent human gonadotropin-?releasing hormone receptor (hGnRH-?R) antagonist.
Synthesis Reference(s)
PROCESS FOR THE PREPARATION OF ELAGOLIX SODIUM AND ITS POLYMORPHThe US patent number 7056927 B2 discloses, elagolix sodium salt as a white solid and process for its preparation in Example-1; Step-IH.
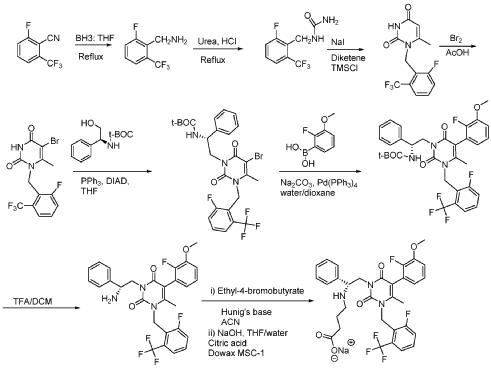
The US patent number 8765948 B2 discloses a process for preparation of amorphous elagolix sodium by spray drying method and solid dispersion of amorphous elagolix sodium with a polymer.
Clinical Use
ORILISSA is indicated for the management of moderate to severe pain associated with endometriosis. ORILISSA 150 mg tablets are light pink, oblong, film-coated tablets with “EL 150” debossed on one side. Each tablet contains 155.2 mg of elagolix sodium (equivalent to 150 mg of elagolix) as the active ingredient and the following inactive ingredients: mannitol, sodium carbonate monohydrate, pregelatinized starch, povidone, magnesium stearate, polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and carmine high tint.

in vitro
Elagolix sodium is a human GnRH receptor (GnRHR) antagonist with an IC50 of 0.25 nM in Kinase assay. Elagolix sodium has advanced to phase 3 trials for the treatment of endometriosis and uterine fibroids. Elagolix sodium also shows NFAT inhibition with an IC50 of 5.4 nM and effectively blocks Ca2+ flux with an IC50 of 0.86 nM[1]. Kinase assay also demonstrates that Elagolix sodium is a human GnRH receptor (GnRHR) antagonist with a Ki value of 3.7 nM[2].