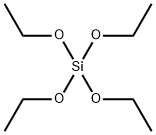
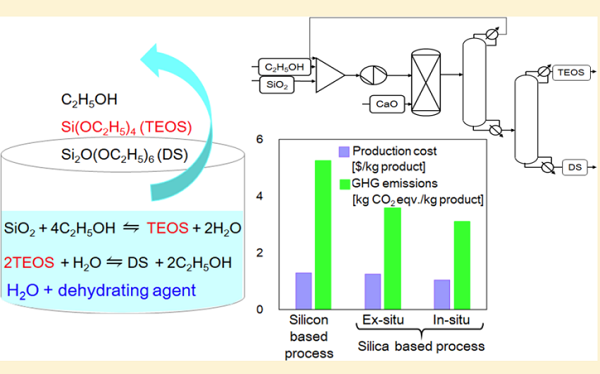
What is Tetraethyl orthosilicate?
Tetraethyl orthosilicate (TEOS) is the chemical compound with the formula Si(OC2H5)4, and it is a colorless liquid that degrades in water. TEOS molecule consists of four ethyl groups attached to SiO4- ion, which is called orthosilicate. As an ion in solution, orthosilicate does not exist. Alternatively TEOS can be considered to be the ethyl ester of orthosilicic acid, Si(OH)4. It is a prototypical alkoxidechemical compound formula orthosilicateethyl esteralkoxide [1].
TEOS is mainly used as a crosslinking agent in silicone polymers and as a precursor to silicon dioxide (SiO2) in the semiconductor industry and is also used as the silica source for the synthesis of some zeolites. When properly hydrolyzed, ethyl silicate produces very fine particles of silica, which can act as a binder to adhere refractories into ceramic shapes or provide corrosion-resistant coatings in combination with zinc dust. Other applications include coatings for carpets and other objects, also used in the production of aerogel. All these applications exploit the reactivity of the Si-OR bonds.
As the most basic chemical of silane, TEOS always acts as the model chemical to show hydrolysis and condensation of silane as shown in scheme 1[2].
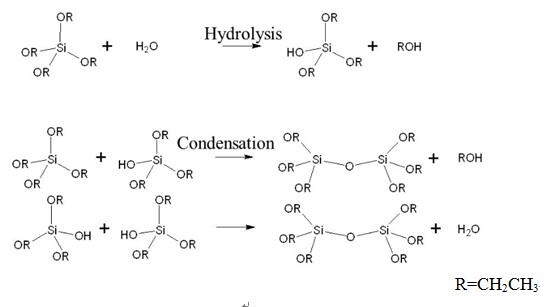
Scheme1 Hydrolysis and Condensation Reaction of TEOS
As shown in scheme 1, the hydrolysis reaction is an example of a sol-gel process and the side product is ethanol. The reaction proceeds via a series of condensation reactions that convert the TEOS molecule into a mineral-like solid via the formation of Si-O-Si linkages. Rates of this conversion are sensitive to the presence of acids and bases, both of which serve as catalysts. At elevated temperatures (>600 °C), TEOS converts to silicon dioxide:
Si(OC2H5)4 → SiO2 + 2O(C2H5)2 (a)
In the equation (a), the volatile coproduct is diethylether [3].
The key to understanding the difference between TEOS and silane is to note that in TEOS the silicon atom is already oxidized: the conversion of TEOS to silicon dioxide is essentially a rearrangement rather than an oxidation reaction, with much reduced changes in free enthalpy and free energy [4].
TEOS is always used with resin to develop hybrid materials in multiapplication. Johannes Karl Fink described the synthesis of a hybrid coating material, which has been synthesized using an acrylate end capped polyester, 1,6-hexanediol diacrylate, tetraethoxysilane TEOS, and 3-trimethoxysilylpropylmethacrylate [5]. The hybrid materials were cast onto a poly(carbonate) (PC) substrate and cured by UV light. In this way, a hybrid film with covalent linkages between the inorganic and the organic networks is formed. The pencil hardness of these materials is higher than 1H, whereas that of an uncoated PC substrate is 6B. The hardness enhancement after coating may be due to the incorporation of the acrylate resin. In another chapter of the same book, an organic-inorganic hybrid interpenetrating network has been synthesized from an epoxide-amine system and tetraethoxysilane (TEOS). In the sol–gel process, hydrolysis and polymerization of TEOS are performed at room temperature in isopropyl alcohol. This results in somewhat smaller silica structures. The most homogeneous hybrid morphology with the smallest silica domains of size 10 to 20 nm can be achieved in a sequential preparation of the interpenetrating network.
References
[1] https://en.wikipedia.org/wiki/Tetraethyl_orthosilicate
[2] https://slideplayer.com/slide/7278894/
[3] https://slideplayer.com/slide/3807805/
[4] http://www.enigmatic-consulting.com/semiconductor_processing/CVD_Fundamentals/films/TEOS_O2_thermal.html
[5] Johannes Karl Fink, Reactive Polymers Fundamentals and Applications, 2nd Edition, 2013
You may like
Related articles And Qustion
Lastest Price from Ethyl silicate manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 78-10-4
- Min. Order:
- 1KG
- Purity:
- 99.5%
- Supply Ability:
- 5000mt
US $0.00/Kg/Drum2025-04-21
- CAS:
- 78-10-4
- Min. Order:
- 25Kg/Drum
- Purity:
- 99%
- Supply Ability:
- 200 tons