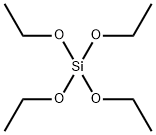
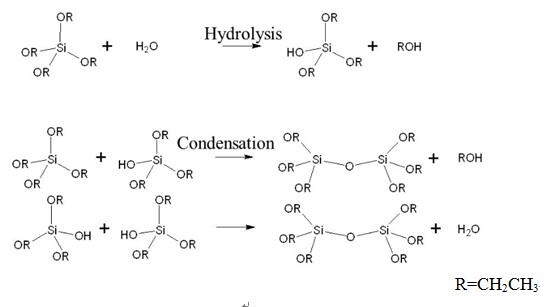
How to synthesize Tetraethyl orthosilicate?
Introduction
Esters of orthosilicic acid of the general formula Si (OR) 4 are widely used in various fields of industry: as a hardener in the creation of organosilicon polymers for dentistry, in jewelry, ceramic manufacturing technology, and in the manufacture of precision casting molds. Alkyl orthosilicates are widely used in chemistry to synthesize highly pure sorbents, various catalyst supports, and airgels. In industry, tetraalkylorthosilicates are prepared by the alcoholysis of toxic silicon tetrachloride in the corresponding alcohol, while the finished alkylorthosilicate is distilled off and the resulting hydrochloric acid is removed. It is known that SiCl 4 is a valuable raw material for the production of high-purity electrochemical silicon for electronics; it is obtained by gas-phase chlorination of a bulk silicon alloy or metallurgical ferrosilicon at 700 °C.
With its moderately reactive Si−OR bonds, tetraethyl orthosilicate or tetraethoxysilane (TEOS) is a valuable ingredient in the synthesis of various silicon compounds, such as zeolite membranes, bifunctional mesoporous organosilica, and siliconnanotube-based batteries. In particular, sol−gel processes use TEOS-based insulators, binders, and crosslinking agents in diverse industries.
Production method
Currently, Tetraethyl orthosilicate is commercially produced from metallurgical silicon (Simg) as the raw material. The synthesis process is well-established, but Simg production from silica-containing sources requires a high temperature (1900 °C) carbon thermal-reduction process, which draws an extremely large electricity supply. Consequently, conventional synthesis is costly to operate and generates high CO2 emissions.
To mitigate these problems, Fukaya's group synthesized TEOS directly from ethanol and silica[1]. This new process reverses the primary reaction at a markedly low chemical equilibrium. The product yield must, therefore, be increased by adding dehydrating agents that remove the water byproduct.
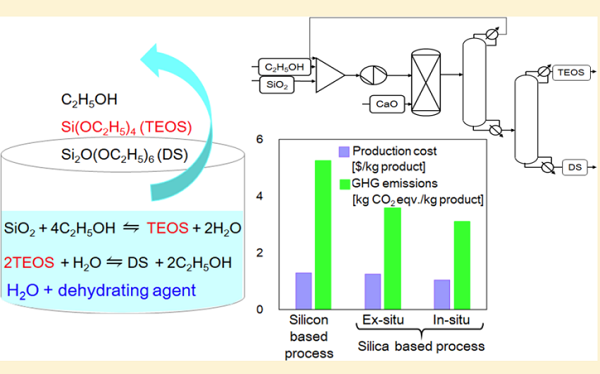
Nguyen et al. compared the performances of in situ water removal from silica-to-TEOS synthesis by different dehydrating agents. CaO was the most suitable water remover among the available dehydrating agents, because it removed water effectively with limited formation of undesired byproducts, thereby reducing the loss of valuable products and raw materials. However, increasing the amount of CaO in the process beyond a certain optimum did not improve the TEOS yield. In this analysis, the most suitable synthesis conditions were a 3:1 molar ratio of CaO to SiO2. Because it requires a much lower residence time and no effort for heat transfer, its process structure is simpler than that of the molecular sieves (MS) process, and so its investment and maintenance costs are lower. Moreover, the reaction product mixture is fed directly to the separation column, reducing the energy requirement for unconverted ethanol recovery. Accordingly, the CaO process is more economically competitive and environmentally friendly than the MS process[2].
The specific process is shown as follows: The Tetraethyl orthosilicate synthesis was carried out directly from silica and ethanol in the presence of a KOH catalyst (10 mol % relative to silica) and different dehydrating agents. The SiO2 (9.0 mmol), KOH (0.9 mmol), dehydrating agent (27 mmol), and EtOH (24 g) were added to a stainless steel autoclave (30 mL inner volume) at room temperature under an N2 atmosphere. The autoclave was heated to 190 °C (the internal temperature was measured with an inserted thermometer) with stirring (400 rpm) for 20 min. The pressure in the autoclave was 2.8 MPa. After cooling to room temperature, p-tert-butyltoluene (70 mg) was added to the mixture as an internal standard. The yields of the product (TEOS) and byproduct (DS) were determined by gas chromatography (GC).
[1] Fukaya, Norihisa et al. “Direct synthesis of tetraalkoxysilanes from silica and alcohols†.” New Journal of Chemistry 6 (2017): 2224–2226.
[2] Thuy T. H. Nguyen*. “Impact of the Water Removal Method on Tetraethyl Orthosilicate Direct Synthesis: Experiment and Process Assessment.” Industrial & Engineering Chemistry Research 58 43 (2019): 19997–20002.
References:
[1] FUKAYA N, CHOI S J, HORIKOSHI T, et al. Direct synthesis of tetraalkoxysilanes from silica and alcohols?[J]. New Journal of Chemistry, 2017, 6: Page 2207 to 2506. DOI:10.1039/C6NJ03897B.[2] THUY T. H. NGUYEN*. Impact of the Water Removal Method on Tetraethyl Orthosilicate Direct Synthesis: Experiment and Process Assessment[J]. Industrial & Engineering Chemistry Research, 2019, 58 43: 19733-20180. DOI:10.1021/acs.iecr.9b02887.
You may like
Related articles And Qustion
See also
Lastest Price from Ethyl silicate manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 78-10-4
- Min. Order:
- 1KG
- Purity:
- 99.5%
- Supply Ability:
- 5000mt
US $0.00/Kg/Drum2025-04-21
- CAS:
- 78-10-4
- Min. Order:
- 25Kg/Drum
- Purity:
- 99%
- Supply Ability:
- 200 tons