What is (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol?
(S) -1- (2,6-dichloro-3-fluorophenyl) ethanol(C8H7C12FO) is synthesized (R)-3-[l-(2, 6-dichloro-3-fluoro-benzene)- The key intermediate of ethoxy-5- (l-piperidine-4-hydroxy-1 hydrogen-pyrazole-4-hydroxy) -pyrimidine-2-indane. And (R)-3-[l-(2,6-dichloro-3-fluoro-benzene)-ethoxy-5-(l-piperidine-4-hydroxy-1 hydrogen-pyrazole-4-hydroxy) -Pyrimidine-2-indane is a small molecule kinase inhibitor for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) positive for anaplastic lymphohematokinase (ALK)[1].
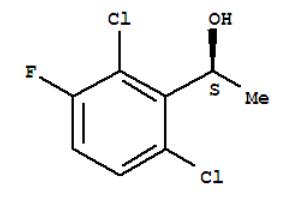
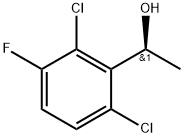
Fig 1. Chemical structure formula of (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol
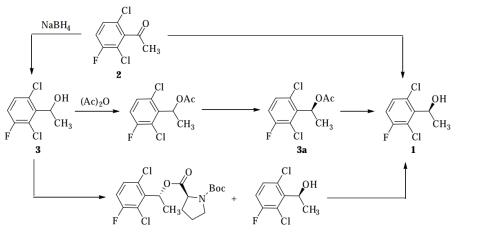
Preparation:There are four ways to synthesize (S) -1- (2,6-dichloro-3-fluorophenyl) ethanol.①Enzyme-catalyzed asymmetric hydrolysis[2,3]: 2,6-dichloro-3-fluoroacetophenone via Reduction of sodium borohydride to obtain racemic 1- (2,6-dichloro-3-fluorophenyl) ethanol, which was esterified with acetic anhydride and then selectively hydrolyzed with pig liver lipase to obtain acetic acid [(S) -1- A mixture of (2,6-dichloro-3-fluorophenyl) ethyl] ester and (R) -1- (2,6-dichloro-3-fluorophenyl) ethanol. The acetic acid obtained by separation and purification [ (S) -1- (2,6-dichloro-3-fluorophenyl) ethyl] ester is further hydrolyzed to obtain (S) -1- (2,6-dichloro-3-fluorophenyl) ethanol, The total yield was 38.9%. This method uses a combination of chemical method and enzymatic method, but the reaction route is long, and requires high enzyme activity and complex process conditions, which limits industrial application.②Enzymatic reduction[3]. Compound 2,6-dichloro-3-fluoroacetophenone was catalyzed by ketone reductase in one step to obtain (S) -1- (2,6-dichloro-3-fluorophenyl) ethanol. The total yield 94%. The method has short reaction steps and high yields, but due to some shortcomings of enzyme reduction, such as unstable catalytic activity, low catalytic efficiency, and difficult control of process conditions, there is still a certain distance from large-scale applications.③Asymmetric catalytic reduction. Compound 2,6-dichloro-3-fluoroacetophenone is reduced by hydrogen under the action of a chiral catalyst (such as SpiroPAP / Ir complex) to obtain (S) -1- (2,6-dichloro-3- Fluorophenyl) ethanol. This method has short reaction steps and only one step reaction, which has good application prospects. However, due to the selection, price and recycling of chiral catalysts, it has not yet achieved industrial application.④Chemical resolution. Compound 2,6-dichloro-3-fluoroacetophenone is reduced with sodium borohydride to give 1- (2,6-dichloro-3-fluorophenyl) ethanol, and then with N-protected chiral amino acid (such as Boc -L-proline) is selectively reacted to an ester, and (S) -1- (2,6-dichloro-3-fluorophenyl) ethanol is finally obtained through operations such as separation and purification, with a total yield of 29.1%. Although this method has long steps, the process conditions are simple and suitable for industrial applications. The main disadvantages are the high price of the resolving agent used, the difficulty in recovery, and the lack of cost competitiveness.
(S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol is an intermediate in the synthetic preparation of Crizotinib (C785000), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor ( c-MET) kinase and anaplastic lymphoma kinase (ALK). Crizotinib is a potential antitumor agent.
References
[1] SAI-HONGI IGNATIUS O, CZNHIA HUANGB B, MARIMK, et al. Crizotinib for the treat ment of ALK- rearranged non-small cell lung cancer: a success story to user in the second decade of molecular targeted therapy in oncollogy. Onlogisticst. 20, 12, 17 (11): 1351-1375.
[2] Kung PP, Martinez CA, Tao JH. Enantioselective biotransformation for preparation of protein tyrosine kinase inhibitor intermediates: WO, 2006021885 [P]. 2006-03-02.
[3] MARTINE CA, KELLE Gen E, MEIJER Min, et al. Biotransformation mediated synthesis of (1S)-l-(2, 6-diclocating) Tetrahdron Asymmetry. 2010, 21(19): 2408-2412.
[4] Liang J, Jenne SJ, Mundorff E, et al. Ketoreductase polypeptides for the reduction of acetophenones: WO, 2009036404 [P]. 2009-03-19.
Related articles And Qustion
See also
Lastest Price from (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol manufacturers
US $0.00-0.00/kg2025-08-29
- CAS:
- 877397-65-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1
US $0.00-0.00/kg2025-08-29
- CAS:
- 877397-65-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1


![127852-28-2 (R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanol;Application; Use](httpss://www.chemicalbook.com/NewsImg/2020-2-24/20202241719318661.jpg)