N,N-Diisopropylethylamine: Application, synthesis and toxicity
General description
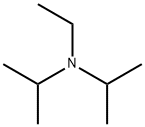
N,N-Diisopropylethylamine (DIPEA) is an organic compound and an amine. It is named after the German chemist Siegfried Hünig. It is used in organic chemistry as a base. N,N-Diisopropylethylamine consists of a central nitrogen that is bonded to an ethyl group and two isopropyl groups. A lone pair of electrons resides on the nitrogen atom, which can react with electrophiles. However, as the two isopropyl groups and the ethyl group occupy much of the space surrounding the nitrogen, only small electrophiles such as protons can react with the nitrogen lone pair. It is traditionally prepared by the alkylation of diisopropylamine with diethyl sulfate. Pure N,N-Diisopropylethylamine exists as a colorless liquid, although commercial samples can be slightly yellow. If necessary, the compound can be purified by distillation from potassium hydroxide or calcium hydride. Its appearance is as follows:

Figure 1 Appearance of N,N-Diisopropylethylamine.
Application
N,N-Diisopropylethylamine is a sterically hindered organic base that is commonly employed as a proton scavenger. Thus, like 2,2,6,6-tetramethylpiperidine and triethylamine, it is a good base but a poor nucleophile, a combination of properties that makes it a useful organic reagent [1]. It is commonly used as the hindered base in amide coupling reactions between a carboxylic acid (typically activated, for example, as an acid chloride) and a nucleophilic amine [2]. As DIPEA is hindered and poorly nucleophilic, it does not compete with the nucleophilic amine in the coupling reaction. DIPEA has been investigated for its use as a selective reagent in the alkylation of secondary amines to tertiary amines by alkyl halides. This is often hampered by an unwanted Menshutkin reaction forming a quaternary ammonium salt, but is absent when DIPEA is present [2].
Moreover, DIPEA can be used as a base in a number of transition metal catalyzed cross-coupling reactions, such as the Heck coupling and the Sonogashira coupling [3]. Besides, DIPEA and triethylamine are structurally very similar, with both compounds considered hindered organic bases. Due to their structural similarity, DIPEA and triethylamine can be used interchangeably in most applications. The nitrogen atom in DIPEA is more shielded than the nitrogen atom in triethylamine. However, triethylamine is a slightly stronger base than DIPEA; the pKas of the respective conjugate acids in dimethyl sulfoxide are 9.0 and 8.5, respectively.
Synthesis
The laboratory experiments were carried out in a 500 ml stirred autoclave from Buchi. Diisopropylamine (DIPA) was placed in the autoclave in which the respective suspended catalyst had been installed. The reactor was flushed with nitrogen. The reactor was pressurized with a low hydrogen pressure and the mixture was heated to the reaction temperature. After reaching the reaction temperature, the hydrogen pressure was increased to the desired reaction pressure. The acetaldehyde was added during the course of the experiment by means of a pump at the reaction pressure within 1 to 5 hours (semibatch mode). Samples were taken from the reactor regularly and analyzed by gas chromatography. The added amount of aldehyde was measured, so that a DIPA conversion achieved was at least 95 %, in total, a molar acetaldehyde-excess resulted in 20 %. The stirrer speed was from 300 to 1200 r/m. The reaction temperature was 100°C and the reaction pressure was 25 bar. Carbon-supported, palladium-containing suspended catalysts with 5 % of Pd on carbon (water content about 50 %) were used as catalyst. A specific amount of catalyst of 0.03 g (catalyst, calc. 100 %)/g (diisopropylamine) was used, i.e. as 6 wt% of water-moist catalyst, based on DIPA. Reaction time -6h. The yield of N,N-Diisopropylethylamine is 67%.
Toxicity
After oral administration of rats, the highest concentration of N,N-Diisopropylethylamine was found in the liver, followed closely by the kidney and spleen. Accumulation was also observed in the heart, lung, and brain but at lower concentrations. Minimizing side effects is a major goal of potential chemotherapeutics considering current treatments such as cisplatin have severe side effects, especially renal toxicity [5].
[2]Dunetz, Joshua R.; Magano, Javier; Weisenburger, Gerald A. (2016). Large-Scale Applications of Amide Coupling Reagents for the Synthesis of Pharmaceuticals. Organic Process Research & Development. 20 (2): 140–177.
[3]Chinchilla, Rafael; Nájera, Carmen (2011). "Recent advances in Sonogashira reactions". Chemical Society Reviews. 40 (10): 5084.
[4]Lepore, Salvatore D.; Khoram, Anita; Bromfield, Deborah C.; Cohn, Pamela; Jairaj, Vinod; Silvestri, Maximilian A. (2005). "Studies on the Manganese-Mediated Isomerization of Alkynyl Carbonyls to Allenyl Carbonyls. The Journal of Organic Chemistry. 70 (18): 7443–7446.
[5]Shelton (2016). Synthesis, anti-proliferative activity, and toxicity of C4(C5) substituted N,N0-bis(arylmethyl)imidazolium salts. Tetrahedron, 72: 5729-5743.
You may like
Related articles And Qustion
Lastest Price from N,N-Diisopropylethylamine manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7087-68-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 7087-68-5
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons