What is Methanesulfonyl chloride?
Physical and chemical properties
Methanesulfonyl chloride is also called methyl sulfonyl chloride, mesyl chloride, methanesulfonic acid chloride. Methanesulfonyl chloride has a melting point of -33ºC and a boiling point of 161ºC. Methanesulfonyl chloride is a colorless transparent liquid, soluble in ethanol and ether, slightly soluble in water, and corrosive. Methanesulfonyl chloride is a colorless oily liquid, and the industrial product is yellowish in color. It is highly corrosive and tear-resistant.
Preparation
There are three preparation methods of methylsulfonyl chloride [1]. The first is made from thiourea by methylation and chlorine oxidation. The second is made by the reaction of methanesulfonic acid and sulfoxide. The third is made from methyl mercaptan obtained by wet chlorination. At present, in China, sulfur vein is basically used as a raw material, and methylsulfonyl chloride is produced by a process route after methylation with dimethyl sulfate and reaction with chlorine gas. The total yield of the reaction is only about 65%. The product needs to be distilled under reduced pressure to meet the general quality requirements, and it has a great corrosion effect on the equipment. Foreign countries generally use methyl mercaptan or dimethyl disulfide as raw materials to react with chlorine, and use hydrochloric acid as the reaction medium.
Specific applications
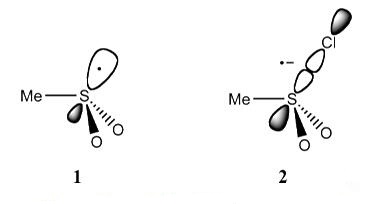
Fig 1. Reduction of sulfonyl chloride in the presence of oxygen [3].
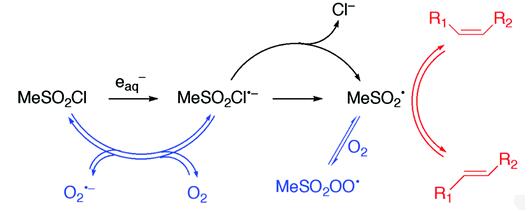
Fig 2. One-electron reduction of methanesulfonyl chloride.[3]
Methanesulfonyl chloride has been used in many fields, such as raw materials in fine chemicals production, catalyst, analytical agent and so on. Specific uses are as following [3-6]:
• Methanesulfonyl chloride reacts easily with compounds with active hydrogen, so it can be used to react with amino and hydroxyl groups to introduce methanesulfonyl.
• As raw material for methanesulfonic acid production.
• The prepared methanesulfonamide is an important intermediate for fine chemical products and can be widely used as a raw material for the synthesis of medicines and pesticides.
• As an analytical reagent.
• As catalyst for esterification and polymerization reaction, stabilizer for liquid sulfur dioxide, chlorinating agent for anthraquinone and carbazole vat dyes, quick-drying agent for dry oil inks and coatings, dyeing improver for polyesters, color adjustment of color photos Agent, condensation agent of xylene, naphthalene and formaldehyde, dyeing aid for wool, etc.
• Raw materials for medicine and pesticides.
References
[1] Noller C R, Hearst P J. Preparation of methanesulfonyl chloride[J]. Journal of the American Chemical Society, 1948, 70(11): 3955-3955.
[2] Giolito S L, Hofmann H O. Methane sulfonyl chloride and process of preparation: U.S. Patent 3,993,692[P]. 1976-11-23.
[3] Tamba M, Dajka K, Ferreri C, et al. One-Electron Reduction of Methanesulfonyl Chloride. The Fate of MeSO2Cl•-and MeSO2• Intermediates in Oxygenated Solutions and Their Role in the Cis− Trans Isomerization of Mono-unsaturated Fatty Acids [J]. Journal of the American Chemical Society, 2007, 129(28): 8716-8723.
[4] Ayers T A, Choy N, Gill H S, et al. Process for the preparation of benzimidazol thienylamine compounds and derivatives thereof useful as sodium/proton exchanger type 3 inhibitors: U.S. Patent Application 12/637,096[P]. 2010-7-8.
[5] Engel R. Methods for Fabrication of Antimicrobial Surfaces: U.S. Patent Application 12/083,478[P]. 2009-9-17.
[6] Gyollai V, Szabo C. Methods of preparing pimecrolimus: U.S. Patent 7,279,571[P]. 2007-10-9.


