What is Imipenem?
Feb 19,2020
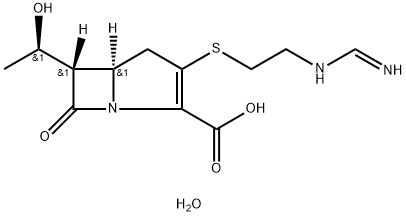
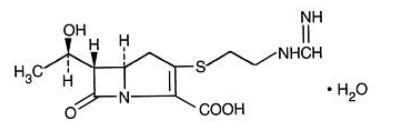
Imipenem (N-formimidoylthienamycin monohydrate) is a crystalline derivative of thienamycin, which is produced by Streptomyces cattleya. Its chemical name is (5 R,6S)-3-[[2-(formimidoylamino)ethyl]thio]-6-[( R)-1-hydroxyethyl ]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monohydrate. It is an off-white, nonhygroscopic crystalline compound with a molecular weight of 317.37. It is sparingly soluble in water and slightly soluble in methanol.Its empirical formula is C12H17N3O4S · H2O, and its structural formula is:
Imipenem is an intravenous β-lactam antibiotic discovered by Merck scientists Burton Christensen, William Leanza, and Kenneth Wildonger in the mid-1970s. Carbapenems are highly resistant to the β-lactamase enzymes produced by many multiple drug-resistant Gram-negative bacteria, thus play a key role in the treatment of infections not readily treated with other antibiotics.
Imipenem was patented in 1975 and approved for medical use in 1985. It was discovered via a lengthy trial-and-error search for a more stable version of the natural product thienamycin, which is produced by the bacterium Streptomyces cattleya. Thienamycin has antibacterial activity, but is unstable in aqueous solution, so impractical to administer to patients. Imipenem has a broad spectrum of activity against aerobic and anaerobic, Gram-positive and Gram-negative bacteria. It is particularly important for its activity against Pseudomonas aeruginosa and the Enterococcus species. It is not active against MRSA, however[1,2].
Imipenem is rapidly degraded by the renal enzyme dehydropeptidase 1 when administered alone, and is almost always coadministered with cilastatin to prevent this inactivation.
Common adverse drug reactions are nausea and vomiting. People who are allergic to penicillin and other β-lactam antibiotics should take caution if taking imipenem, as cross-reactivity rates are high. At high doses, imipenem is seizurogenic.Imipenem acts as an antimicrobial through inhibiting cell wall synthesis of various Gram-positive and Gram-negative bacteria. It remains very stable in the presence of β-lactamase (both penicillinase and cephalosporinase) produced by some bacteria, and is a strong inhibitor of β-lactamases from some Gram-negative bacteria that are resistant to most β-lactam antibiotics[3,4].
Imipenem is a semisynthetic thienamycin that has a wide spectrum of antibacterial activity against gram-negative and gram-positive aerobic and anaerobic bacteria, including many multiresistant strains.It is stable to many beta-lactamases. Similar compounds include meropenem, known for having greater activity against Gram negative bacteria, and the newer ertapenem which exhibits a longer half-life due to increased binding to plasma proteins.10 Imipenem is commonly used in combination with cilastatin and is now available in a triple-drug product with cilastatin and relebactam which was recently approved by the FDA. Imipenem was first approved by the FDA in November 1985 as the combination product Primaxin marketed by Merck & Co.Imipenem is indicated, in combination with cilastatin with or without relebactam, for the treatment of bacterial infections including respiratory, skin, bone, gynecologic, urinary tract, and intra-abdominal as well as septicemia and endocarditis.Imipenem is a beta-lactam antibiotic belongings to the subgroup of carbapenems. Imipenem is active against aerobic and anaerobic Gram positive as well as Gram negative bacteria including Pseudomonas aeruginosa and the Enterococcus. It exerts a bactericidal effects by disrupting cell wall synthesis.
Imipenem acts as an antimicrobial through the inhibition of cell wall synthesis of various gram-positive and gram-negative bacteria.This inhibition of cell wall synthesis in gram-negative bateria is attained by binding to penicillin-binding proteins (PBPs). In E. coli and selected strains of P. aeruginosa, imipenem has shown to have the highest affinity to PBP-2, PBP-1a, and PBP-1b. This inhibition of PBPs prevents the bacterial cell from adding to the peptidoglycan polymer which forms the bacterial cell wall eventually leading to cell death[5-7].
References
[1]Clissold, SP; Todd, PA; Campoli-Richards, DM (Mar 1987). "Imipenem/cilastatin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy". Drugs. 33 (3): 183–241.
[2]Vardakas, KZ; Tansarli, GS; Rafailidis, PI; Falagas, ME (Dec 2012). "Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis". The Journal of Antimicrobial Chemotherapy. 67 (12): 2793–803.
[3]Kahan, FM; Kropp, H; Sundelof, JG; Birnbaum, J (Dec 1983). "Thienamycin: development of imipenen-cilastatin". The Journal of Antimicrobial Chemotherapy. 12 Suppl D: 1–35.
[4]Kesado, Tadataka; Hashizume, Terutaka; Asahi, Yoshinari (1980). "Antibacterial activities of a new stabilized thienamycin, N-formimidoyl thienamycin, in comparison with other antibiotics". Antimicrobial Agents and Chemotherapy. 17 (6): 912–7.
[5]J. N. Kattan, M. V. Villegas, J. P. Quinn. New developments in carbapenems[J]. Clin Microbiol Infect, 14(12):1102-1111.
[6]DA Pastel. Imipenem-cilastatin sodium, a broad-spectrum carbapenem antibiotic combination[J]. Clin Pharm, 1986, 5(9):719-736.
You may like
Application research of Lactitol
Feb 5, 2026
Application research of Ziconotide acetate
Feb 5, 2026
Application research of Policresulen
Jan 29, 2026
See also
Lastest Price from Imipenem manufacturers
Imipenem

US $2.00-6.00/kg2025-07-25
- CAS:
- 74431-23-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg
Imipenem
US $0.00/kg2025-04-14
- CAS:
- 74431-23-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS