ChemicalBook > Articles Catagory List >Heterocyclic-compounds >what-is-5-bromopyridine-2-carboxylic-acid-
What is 5-Bromopyridine-2-carboxylic acid?
Feb 19,2020
5-Bromopyridine-2-carboxylic acid is used as a pharmaceutical intermediate. Store in cool, dry place in a well sealed container. Store away from oxidizing agents.
5-Bromopyridine-3-carboxylic acid (5-bromonicotinic acid) was used in the synthesis of 3-guanidinomethyl-5-iodopyridine.
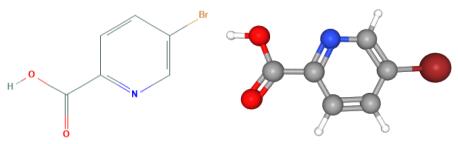
Fig 1. Chemical structure formula and three-dimensional structure of 5-Bromopyridine-2-carboxylic acid
Reactions of mono-, di-, tri-alkyltin chlorides or oxide with 5-bromopyridine-2-carboxylic acid result in five new organotin(IV) compounds, [MeSn(O2CC5NH3Br)Cl2(H2O)]·(C2H5)2O, [(n-Bu)Sn(O2CC5NH3Br)Cl2(H2O)]·(C2H5)2O, {[(n-Bu)2Sn(O2CC5NH3Br)]2O}2 [(n-Bu)3Sn(O2CC5NH3Br)]n and [Ph3Sn(O2CC5NH3Br)]n.
Studies reveal that the introduction of halogen atoms into the antitumor agents will have a significan affect on their biological activity. As part of our interest to pyridine carboxylic acid ligands, we intend to modify the composition of the organotin(IV) 5-bromopyridine-2-carboxylates with mono-, bi-, tri-alkyltin salts, as well as adjust different coordination solvent molecules[1-4].
Synthesis of compound [MeSn(O2CC5NH3Br)Cl2(H2O)]·(C2H5)2O: The reaction was carried out under nitrogen atmosphere with the use of the the standard Schlenk technique. 5-Bromopyridine-2-carboxylic acid (0.202g, 1 mmol) and sodium ethoxide (0.068 g, 1.0 mmol) were added to 20 mL absolute benzene and heated under reflux with stirring for 0.5 h. After the addition of methyltin trichloride (0.24 g, 1.0 mmol) to the reactor, the reaction mixture was refluxed for 10 h more. The reaction solution obtained after filtering was evaporated in vacuum to give a solid, which was then recrystallized from diethyl/petroleum ether to give colorless block crystals suitable for single crystal X-ray diffraction. Yield: 76%.
Synthesis of compound [(n-Bu)Sn(O2CC5NH3Br)Cl2(H2O)]·(C2H5)2O:5-Bromopyridine-2-carboxylic acid (0.202 g, 1 mmol) and sodium ethoxide (0.068 g, 1.0 mmol) were added to dry benzene (20 ml) in a Schlenk flask and stirred for 0.5 h. n-Butyltin trichloride (0.28 g, 1.0 mmol) was then added and the reaction mixture was refluxed for 10 h more and then filtered. The solvent was gradually removed by evaporation under vacuum until a solid product was obtained. The solid was then recrystallized from diethyl/petroleum ether to give colorless crystals suitable for single crystal X-ray diffraction were obtained. Yield 71%.
Synthesis of compound {[(n-Bu)2Sn(O2CC5NH3Br)]2O}2:5-Bromopyridine-2-carboxylic acid (0.202g,1 mmol) was added to benzene solution (30 ml) of di-n-butyltin oxide (0.2487g,1 mmol). The mixture was heated under reflux with stirring for 10 h. The clear solution thus obtained was evaporated under vacuum to form a solid, and recrystallized in dichloromethane/hexane. Then the colorless crystals suitable for single crystal X-ray diffraction were obtained. Yield: 85%.
Synthesis of compound [(n-Bu)3Sn(O2CC5NH3Br)]n:5-bromopyridine-2-carboxylic acid (0.202 g, 1.0 mmol) and sodium ethoxide (0.068 g, 1.0 mmol) were added to dry benzene (20 ml) and stirred for 0.5 h. Tri-n-butyltin chloride (0.325 g, 1.0 mmol) was then added and the reaction mixture was refluxed for 10 h more and then filtered. The solvent was gradually removed by evaporating under vacuum until a solid product was obtained. The solid was then recrystallized from diethyl/petroleum ether and colorless block crystals suitable for X-ray diffraction were obtained. Yield: 72%.
Synthesis of compound [Ph3Sn(O2CC5NH3Br)]n:5-bromopyridine-2-carboxylic acid (0.202g, 1.0 mmol) and sodium ethoxide (0.068g, 1.0 mmol) were added to dry benzene (20 ml) and stirred for 0.5h. Triphenyltin chloride (0.385g, 1.0 mmol) was then added and the reaction mixture was refluxed for 10h more and then filtered. The solvent was gradually removed by evaporating under vacuum until a solid product was obtained. The solid was then recrystallized from diethyl/petroleum ether and colorless block crystals suitable for X-ray diffraction were obtained. Yield: 83%.
References
[1]Yang X, Fu, Boqiao, Yu, Biao. Total Synthesis of Landomycin A, a Potent Antitumor Angucycline Antibiotic[J]. Journal of the American Chemical Society, 133(32):12433-12435.
[2]Hazeldine, Stuart T, Polin, Lisa, Kushner, Juiwanna. II. Synthesis and Biological Evaluation of Some Bioisosteres and Congeners of the Antitumor Agent, 2-{4-[(7-Chloro-2-quinoxalinyl)oxy]phenoxypropionic Acid (XK469)[J]. Journal of Medicinal Chemistry, 45(14):3130-3137.
[3]Pan, Xiaogang, Chen, Li, Liu, Shujun, Yang, Xiaojuan, Gao, Jian-Xin, & Lee, Robert J. . Antitumor activity of g3139 lipid nanoparticles (lnps). Molecular Pharmaceutics, 6(1), 211-220.
[4]Hong M , Yin H D , Zhang Y W , et al. Coordination geometry of monomeric, dimeric and polymeric organotin(IV) compounds constructed from 5-bromopyridine-2-carboxylic acid and mono-, di- or tri-organotin precursors[J]. Journal of Molecular Structure, 2013, 1036:244-251.
Lastest Price from 5-Bromo-2-pyridinecarboxylic Acid manufacturers
5-Bromo-2-pyridinecarboxylic Acid

US $0.00-0.00/KG2025-05-29
- CAS:
- 30766-11-1
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KGS
5-Bromopyridine-2-carboxylic acid

US $10.00/KG2025-04-21
- CAS:
- 30766-11-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt