What is Ectoine?
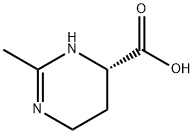
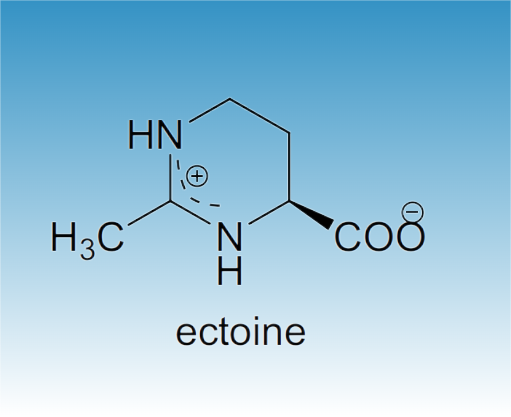
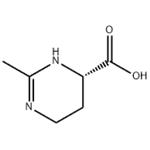
Ectoine (1,4,5,6-tetrahydro-2-methyl-4-pyrimidinecarboxylic acid) is a natural compound found in several species of bacteria. It is a compatible solute which serves as a protective substance by acting as an osmolyte and thus helps organisms survive extreme osmotic stress. Ectoine is found in high concentrations in halophilic microorganisms and confers resistance towards salt and temperature stress. Ectoine was first identified in the microorganism Ectothiorhodospira halochloris, but has since been found in a wide range of Gram-negative and Gram-positive bacteria.
Features
Ectoine, a small organic molecule, occur widely in aerobic, chemoheterotrophic, and halophilic organisms that enable them to survive under extreme conditions. These organisms protect their biopolymers (bio membranes, proteins, enzymes, and nucleic acids) against dehydration caused by high temperature, salt concentration, and low water activity by substantial ectoine synthesis and enrichment within the cell. The organic osmolyte, ectoine and hydroxyectoine are amphoteric, water-binding, organic molecules. They are generally compatible with the cellular metabolism without adversely affecting the biopolymers or physiologic processes and are so-called compatible solutes.small organic molecule.
Like other compatible solutes, ectoine and 5-hydroxyectoine are low-molecular mass compounds that are highly soluble in water (about 4 M at 20℃), thereby allowing the amassing of these compounds to near molar concentrations in severely osmotically stressed microbial cells. A variety of biophysical techniques have been used to study the effects of ectoine on the hydration of proteins and cell membranes and on interactions mediated via hydrogen bonding.
Uses
The uses of Ectoine are as follows:Ectoine Stabilizer in biotechnological processes; protects the taste of food during dehydration; moisturizer in cosmetics; protects skin against photoaging and formation of sunburn cells.
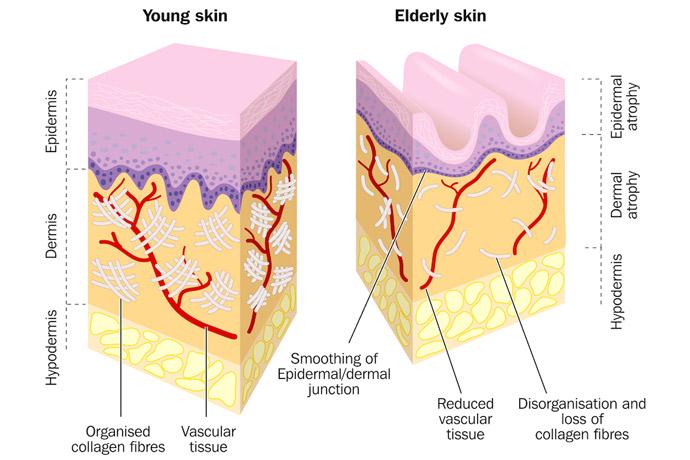
Ectoine in dermatology and cosmetology applications
High temperature, UV radiation, wind, water and air pollution are factors that affect our skin health. Ectoine has commercial uses as an enzyme stabilizer and as a cell protectant in skin care applications. It is widely used in cosmetics, as it speeds up the defence system of the skin and it has a great anti-aging effect by softening and reducing wrinkles. In dermatology, it is often contained in cream preparations for the treatment of skin dryness, roughness and scaliness (atopic dermatitis, psoriasis), UV-induced sunburn cells and overall skin dehydration.
Ectoine in nasal sprays and the treatment of eye disorders
Thanks to its lubricating effect, ectoine is also administered for the treatment of eye disorders. It relieves irritation and inflammation by stabilizing the watery layer and the lipid layer of the tear film, ensuring optimal lubrication. Ectoine increases the binding of water with the cells of the ocular surface acting as a mechanism against allergenic substances and providing relief and improvement of the disturbing allergic symptoms.
Ectoine is also used in nasal sprays to treat symptoms of respiratory impairment, induced by carbon-related lung inflammation, allergic rhinitis or asthma.
Ectoine for the prevention of chemotherapy-induced oral mucositis
In recent pilot studies, ectoine was tested for the prevention and treatment of chemotherapy-induced oral mucositis, especially in patients with head and neck squamous cell carcinoma (HNSCC) (Nature, (2019) 9:6594).
Ectoine is synthesized in three successive enzymatic reactions starting from aspartic β-semialdehyde. The genes involved in the biosynthesis are called ectA, ectB and ectC, and they encode the enzymes L-2,4-diaminobutyric acid acetyltransferase, L-2,4-diaminobutyric acid transaminase and L-ectoine synthase, respectively.
Ectoine is synthesized from aspartate-semialdehyde, the central intermediate in the synthesis of amino acids belonging to the aspartate family. Ectoine formation comprises three enzymatic steps. First, aspartate-semialdehyde is transaminated to 2,4-diaminobutyric acid (DABA) with glutamate as amino-group donor. The transamination is catalyzed by DABA transaminase ectB. EctB is a 421-residue protein with a molecular mass of 46.1 kDa, which requires K 1 for its transaminase activity and for protein stability. Gel filtration experiments with purified protein from H. elongata indicate that the DABA aminotransferase ectB might form a homohexamer in the native state. Then, an acetyl group is transferred to DABA from acetyl-CoA by DABA-Nγ-acetyltransferase ectA in order to synthesize Nγ-acetyl-L-2,4-diaminobutyric acid. EctA is a 192-residue protein with a calculated molecular mass of 21.2 kDa. Finally, ectoine synthase ectC catalyzes the cyclic condensation of Nγ-acetyl-2,4-diaminobutyric acid, which leads to the formation of ectoine. EctC is a 137-residue protein with a calculated molecular weight of 15.5 kDa with a pI value of 4.9. The ectC protein belongs to the enzyme family of carbon-oxygen lyases. In vitro experiments with purified ectC revealed that ectoine-synthase activity and affinity to its substrate are strongly affected by NaCl. Under certain stress conditions (e.g. elevated temperatures) H. elongata converts some of the ectoine to 5- hydroxyectoine by ectoine hydroxylase (EctD). The ectoine hydroxylase EctD consists of 332 amino acids and has a molecular weight of 37.4 kDa.
You may like
Related articles And Qustion
See also
Lastest Price from Ectoine manufacturers
US $0.00-0.00/kg2025-08-21
- CAS:
- 96702-03-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00-0.00/kg2025-08-19
- CAS:
- 96702-03-3
- Min. Order:
- 1kg
- Purity:
- 99%HPLC
- Supply Ability:
- 20T