What is Favipiravir?
Antiviral drug
Favipiravir is a pyrazine carboxamide derivative developed by Toyama Chemical of Japan to act against many RNA viruses. It was first described as a selective inhibitor of influenza virus replication with minimal cytotoxicity (Furuta et al., 2002). It is believed to directly target the RdRp catalytic site preventing virus replication in cells and inhibiting infection; it has been shown to have in vitro and/or in vivo antiviral activity against a wide array of human-infecting RNA viruses, including ss(–)RNA viruses.
The drug is converted intracellularly into its active, phosphoribosylated form, which is recognized as a substrate by the viral RNA-dependent RNA polymerase. Interestingly, besides its anti-influenza virus activity, this molecule is also able to inhibit the replication of flavi-, alpha-, filo-, bunya-, arena-, noro-, and of other RNA viruses, which include neglected and (re)emerging viruses for which no antiviral therapy is currently available.

Favipiravir is a broad-spectrum antiviral drug that was cleared by the Drugs Controller General of India (DCGI) last week for “emergency restricted” use among Covid-19 patients. Glenmark will sell the generic favipiravir under the brand name FabiFlu.
Favipiravir was originally developed in the late 1990s by a company that was later purchased by the Japanese firm Fujifilm as part of its transition from the photo business to healthcare.
After being tested against a range of viruses, the drug was approved in Japan in 2014 for emergency use against flu epidemics or to treat new strains of influenza.
Mode of Action
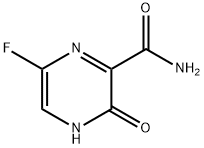
Favipiravir (T-705; 6-fluoro-3-hydroxy-2-pyrazinecarboxamid, Fig. 11.8G) is an antiviral drug that selectively inhibited the RdRP of influenza virus. It showed specific activity against all three influenza A, B, and C (Furuta et al., 2013). It also inhibited the RV replication in HeLa cells, with an EC50 of 29 µg/mL (Furuta et al., 2002). Analysis showed that the primary mechanism of action of favipiravir against the influenza virus was specific inhibition of vRNA polymerase (Furuta et al., 2005). It is predicted that a similar mechanism might occur with other viruses, such as PV and RV, inhibited by favipiravir, which may account for its broad-spectrum inhibition. Mechanistic studies show that the favipiravir and its form favipiravir-RMP (favipiravir-ribofuranosyl-50-monophosphate) do not inhibit influenza RNA polymerase activity, but it is the phosphoribosylated form, favipiravir-ribofuranosyl-50-triphosphate (RTP) that inhibits the enzyme.
Metabolism of favipiravir to its triphosphate form occurs in an extracellular environment in a concentration-dependent manner. The vRNA polymerase mistakenly recognizes favipiravir-RTP as a purine nucleotide. This favipiravir-RTP is misincorporated in nascent vRNA, or it may act by binding to conserved polymerase domains, preventing incorporation of nucleotides for vRNA replication and transcription (Jin et al., 2013).
Clinical trials in India
Glenmark started a randomised, multi-centric study involving 150 patients to test favipiravir among Covid-19 patients in India on 20 May. The study was carried out at 11 sites, and the results sought to draw a comparison between treatment with favipiravir and that involving standard care.
The trial ended on 16 June, but the results have not yet been made public. Glenmark is conducting a second clinical trial that uses favipiravir in combination with umifenovir — another antiviral drug that blocks the entry of SARS-CoV-2 into the human cells.
In this trial, about 158 Covid-19 patients will be divided into two groups The team will test the efficacy of the drug combination in comparison to treatment with favipiravir alone in mild to moderate Covid-19 patients.
A third trial, which will test the efficacy of favipiravir in mild to moderate patients, has been registered by drug manufacturing company Cipla. The multicentre study will evaluate the efficacy and safety of favipiravir along with supportive care in 156 patients with mild to moderate coronavirus infection. Results will be compared against a control group that will only be administered supportive care.
Do Favipiravir and Remdesivir work against Covid-19?
Among the many drugs that the Drug Controller General of India has been approving for the treatment of Covid-19, two anti-viral drugs Favipiravir for those with mild to moderate symptoms of coronavirus in Covid-19 patients and Remdesivir for Covid-19 patients on oxygen support have been at the forefront.
Favipiravir was launched recently by Glenmark Pharmaceuticals, Cipla and Hetero introduced Remdesivir. Many experts, however, have raised concerns on its efficacy. For instance, both these drugs were originally designed to treat other diseases such as any skin disease or arthritis.
Similarly, Remdesivir is an experimental anti-viral drug, which is being studied for Ebola. Now, though, the companies have introduced the drugs’ generic version to treat coronavirus patients, especially Remdesivir being used on compassionate grounds to counter Covid-19.Dr Reddy’s Laboratories Ltd. (DRL), a global pharmaceutical company headquartered in India, is scaling up antimalarial drug hydroxychloroquine, which is now being used as a prophylactic drug for Covid-19.
However, the positive results from the study should be interpreted with caution as other therapies were administered in this non-randomised, open-label study, which could have confused the results.
“Though a multinational, randomised placebo-controlled trial observed reduced time to recovery from severe Covid-19 with use of Remdesivir, another study conducted in China reported conflicting results. The drug shouldn’t be used in patients having hypersensitivity to any ingredients of the formulation, patients with gross liver enzyme and renal function abnormalities.
With this drug-repurposing, we get accustomed to thinking that such drugs are all antiviral, which may not be the case. Favipiravir, for instance, was made in Japan about 13 years ago for influenza. Doctors using Favipiravir in the trials for Covid-19 observed that the drug suppressed the symptoms in people suffering from mild attacks of corona. However, the drug does not offer total safety and the disease may recur in the patient. There can be grave side effects too some people die with these drugs!
A Potential Antiviral for COVID-19?
Currently, there is not any specific effective antiviral treatment for COVID-19. Although most of the COVID-19 patients have mild or moderate courses, up to 5%–10% can have severe, potentially life threatening course, there is an urgent need for effective drugs. Optimized supportive care remains the mainstay of therapy. There have been more than 300 clinical trials going on, various antiviral and immunomodulating agents are in various stages of evaluation for COVID-19 in those trials and some of them will be published in the next couple of months. Despite the urgent need to find an effective antiviral treatment for COVID-19 through randomized controlled studies, certain agents are being used all over the world based on either in-vitro or extrapolated evidence or observational studies. The most frequently used agents both in Turkey and all over the world including chloroquine, hydroxychloroquine, lopinavir/ritonavir, favipiravir and remdesivir will be reviewed here .Nitazoxanide and ivermectin were also included in this review as they have recently been reported to have an activity against SARS-CoV-2 in vitro and are licensed for the treatment of some other human infections.
You may like
Related articles And Qustion
See also
Lastest Price from Favipiravir manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 259793-96-9
- Min. Order:
- 10g
- Purity:
- 99%min HPLC
- Supply Ability:
- 10KGS

US $25.00/ASSAYS2025-04-21
- CAS:
- 259793-96-9
- Min. Order:
- 100ASSAYS
- Purity:
- 99.5%
- Supply Ability:
- 100 mt