Favipiravir: A Repurposed Antiviral Drug Showing Promising COVID-19 Treatment
General Description
Favipiravir, an oral antiviral drug originally approved in Japan for influenza, has been repurposed for COVID-19 treatment due to its ability to inhibit RNA-dependent RNA polymerase, crucial for coronavirus replication. Demonstrating efficacy in vitro against SARS-CoV-2, it offers a promising outpatient treatment option, especially for mild to moderate cases. Global clinical studies have shown Favipiravir's potential in reducing viral load and improving clinical outcomes, with various countries reporting positive results. However, its effectiveness is influenced by the dosing regimen, with a higher dose suggested for COVID-19 compared to influenza, to address the higher EC50 value. Despite promising data, limitations such as non-randomization and the need for peer-reviewed publications exist.

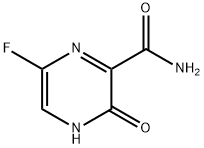
Figure 1. Favipiravir
A repurposed drug for COVID-19
Favipiravir is an oral, broad-spectrum antiviral drug that has been repurposed for the treatment of COVID-19. Originally approved in Japan for new and reemerging pandemic influenza, Favipiravir functions as an inhibitor of RNA-dependent RNA polymerase (RdRp), a key enzyme in the replication machinery of coronaviruses. The drug's mechanism involves being metabolized into its active form inside cells and then incorporated into viral RNA. This process leads to premature termination of the viral replication chain or accumulation of harmful mutations within the virus. Its efficacy against SARS-CoV-2, the virus causing COVID-19, has been demonstrated in vitro, indicating that Favipiravir can achieve effective concentrations against the virus within a safe therapeutic dose range. Given its oral formulation and the predominance of mild to moderate COVID-19 cases, Favipiravir presents a significant advantage in potentially meeting the clinical needs of a large portion of infected individuals who can be treated on an outpatient basis. The COVID-19 task force committee of India has highlighted Favipiravir as one of the most promising drugs, taking into account various factors including scientific evidence, mechanism of action, preclinical results, human safety data, and progress in clinical trials. Early clinical studies from China have reported promising outcomes, showing reductions in viral load and improvements in clinical and radiological findings, underscoring Favipiravir's potential as a beneficial treatment option for COVID-19. 1
Global clinical studies in COVID-19
Global clinical studies on the use of Favipiravir for treating COVID-19 have shown promising results across various countries. In China, an open-label control study comparing Favipiravir with LPV/RTV in patients with mild to moderate COVID-19 demonstrated that Favipiravir led to shorter viral clearance times and significant improvements in chest imaging. Another Chinese trial comparing Favipiravir with umifenovir found that Favipiravir significantly improved clinical recovery rates and shortened the latency for symptom relief without affecting overall respiratory failure rates or mortality. In Japan, a large observational registry reported high rates of clinical improvement among patients with varying disease severities after starting Favipiravir therapy. The majority of these patients received an initial high dose followed by a maintenance dose, with treatment beginning within three days of hospitalization on average. However, hyperuricemia and liver function abnormalities were noted as common adverse events. Russia approved Favipiravir based on early results from an ongoing trial showing faster fever resolution and viral elimination compared to standard care. Similarly, a retrospective observational study from Thailand highlighted clinical improvement with Favipiravir, indicating a lower loading dose as a negative prognostic factor for day-7 clinical outcomes. A recent Japanese trial comparing early versus late Favipiravir administration in hospitalized patients suggested a trend towards better viral clearance and faster defervescence in the early treatment group. These studies collectively underscore Favipiravir's potential as a treatment for COVID-19, although limitations such as non-randomization, open-label designs, and the need for peer-reviewed publication are noted. 2
Dosage
The dosing regimen of Favipiravir, an antiviral drug, plays a crucial role in its effectiveness against viral infections. For influenza treatment in Japan, the approved regimen starts with a loading dose of 3200 mg on the first day, followed by a maintenance dose of 600 mg twice daily from the second to the fifth day. During the Ebola virus disease outbreak, the JIKI trial indicated that a higher dose (6000 mg on day 0 and 2400 mg/day from day 1 to day 9) improved survival rates in patients with moderate to high viral loads. Studies suggest that Favipiravir effectively reduces viral load in cases of medium to high viremia. Considering COVID-19, a higher dose is recommended due to Favipiravir's EC50 being higher than that for influenza. The current suggested dosage for COVID-19 treatment involves an 1800 mg loading dose twice on the first day, followed by 800 mg twice daily up to a maximum of 14 days, to potentially impact viral load reduction. 2
Reference
1. Shannon A, Selisko B, Le NT, et al. Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis. Nat Commun. 2020;11(1):4682.
2. Joshi S, Parkar J, Ansari A, et al. Role of favipiravir in the treatment of COVID-19. Int J Infect Dis. 2021;102:501-508.
Related articles And Qustion
See also
Lastest Price from Favipiravir manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 259793-96-9
- Min. Order:
- 10g
- Purity:
- 99%min HPLC
- Supply Ability:
- 10KGS

US $25.00/ASSAYS2025-04-21
- CAS:
- 259793-96-9
- Min. Order:
- 100ASSAYS
- Purity:
- 99.5%
- Supply Ability:
- 100 mt