What is Anisole used as?
What is anisole?
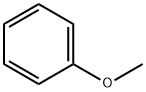
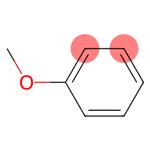
Anisole, also known as methoxybenzene (PhOMe), is a versatile organic aromatic ether compound. It is weakly polar, insoluble in water, and soluble in organic solvents such as alcohols and ethers. PhOMe has a functional methoxy group (-OCH3) on the benzene ring, has an aromatic odor, and is commonly used as a chemical solvent and an intermediate product in organic synthesis.
Uses
Hydrogenation reaction of anisole
Anisole can be efficiently converted to cyclohexane over Ni/Al2O3 catalysts. It can be used as a model molecule to study the hydrodeoxygenation (HDO) of lignin-derived oxygenated compounds. Anisole adsorbs on the acidic sites of Al2O3, and the adsorption at 200-300°C is reactive in nature and leads to its demethylation. Studies on the initial reaction stage show that the HDO of anisole on Ni/Al2O3 catalysts proceeds through two independent pathways, namely reactive adsorption/(de)methylation and aromatic ring hydrogenation.
Biofuels
Anisole is a potential source of biofuels made from cellulose-based compounds. It is mainly used as a substitute for phenol-rich compounds.
Perfumes and Fragrances
Due to its aromatic smell, anisole can be used to synthesize other aromatic compounds such as anethole. It is used as a food flavoring and perfume source.
In addition, anisole binary complex is also a typical system for studying ground state and electronic excited state microsolvation.
Synthesis
Phenol can be treated with sodium hydroxide and then reacted with methyl bromide to form anisole. This method involves a two-step reaction process:
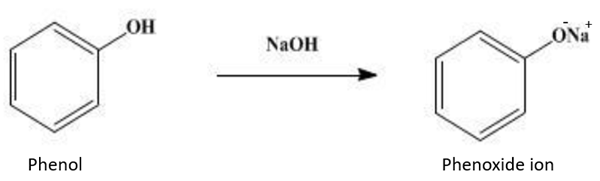
(1) Formation of phenoxide ion. When phenol is treated with sodium hydroxide, it forms phenoxide ion:
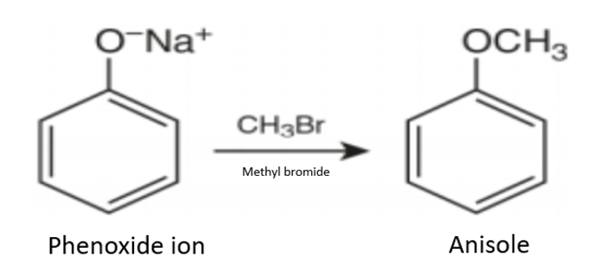
(2) The formed phenoxide ion reacts with methyl bromide to form anisole
References:
[1] SNEHASIS DUTTA . Evaluation of anisole hydrodeoxygenation reaction pathways over a Ni/Al2O3 catalyst[J]. Journal of Catalysis, 2024, 435. DOI:10.1016/j.jcat.2024.115553.[2] AGNIESZKA KRZEMI?SKA. Anisole-Water and Anisole-Ammonia Complexes in Ground and Excited (S1) States: A Multiconfigurational Symmetry-Adapted Perturbation Theory (SAPT) Study.[J]. The Journal of Physical Chemistry A, 2024. DOI:10.1021/acs.jpca.4c04928.
[3] OLEG KIKHTYANIN . Inhibiting effects during the co-conversion of lauric acid and anisole over Ni and NiMo catalysts[J]. Applied Catalysis A: General, 2024, 685. DOI:10.1016/j.apcata.2024.119889.
[4] ROY S, ASKARI O. Chemical Kinetic Study on Reaction Pathway of Anisole Oxidation at Various Operating Condition[J]. Volume 8: Energy, 2020, 303 1: 156-162. DOI:10.1115/IMECE2020-24233.
Related articles And Qustion
See also
Lastest Price from Anisole manufacturers
US $0.00/kg2025-04-15
- CAS:
- 100-66-3
- Min. Order:
- 20kg
- Purity:
- 99%
- Supply Ability:
- 20 tons

US $10.00/KG2025-03-07
- CAS:
- 100-66-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt