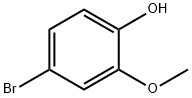
What is 4-Bromo-2-methoxyphenol?
Feb 11,2020
4-bromo-2-methoxyphenol is an important organic intermediate (building block) to synthetize substituted phenzyl products, and it can also be used for the synthesis of polyfunctionalized bicyclic systems.
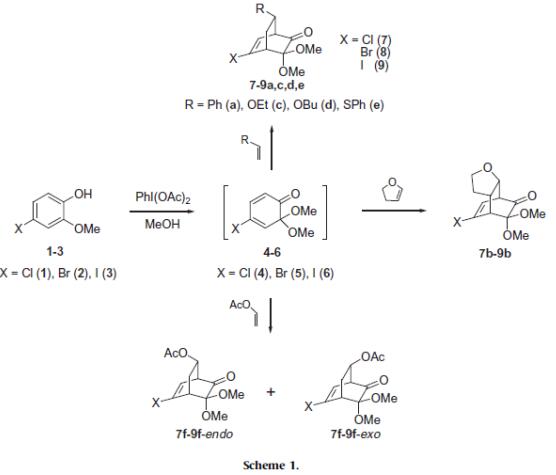
Surasani et al. reported [1] its application on the Diels–Alder reaction of 4-halogenated masked o-benzoquinones with electron-rich dienophiles. Inverse-electron-demand Diels–Alder reaction of masked o-benzoquinones (MOBs) ensuing from the corresponding 4-halo-2-methoxyphenols with styrene, dihydrofuran and ethyl vinyl ether, butyl vinyl ether, phenyl vinyl sulfide and vinyl acetate afford the highly functionalized halogen substituted bicylclo[2.2.2]octenones. This method demonstrates the synthesis of polyfunctionalized bicyclic systems from relatively less reactive dienophiles and stable 4-halo MOBs.
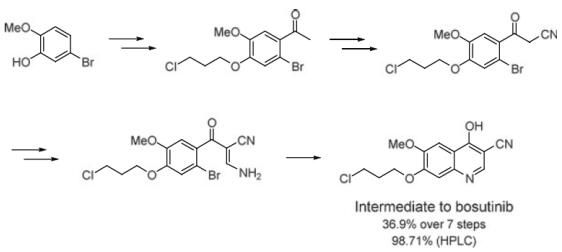
Zhu et al. reported [2] its application on the synthesis of 7-(3-chloropropoxy)-4- hydroxy-6-methoxyquinoline-3- carbonitrile, which is a Key Intermediate to Bosutinib. 5-Bromo-2-methoxyphenol is adopted as the starting material via the simple chemical process including Friedel-Crafts reaction, alkylation, bromination, cyano substitution, and so on to give the 3-amino-2-(2-bromobenzoyl)acrylonitrile, which underwent key intramolecular cyclization at K2CO3/DMF condition; the final product was obtained in 36.9% yield over 7 steps and 98.71% purity (HPLC). Bosutinib (SKI-606), marketed as Bosulif, is an adenosine triphosphate (ATP)-competitive Bcr-Abltyrosine-kinase inhibitor with an additional inhibitory effect on SRc family kinases (including Src, Lyn, and Hck) for use in the treatment of cancer. Bosulif was received the US Food and Drug Administration (FDA) and European Union (EU) European Medicines Agency approval on 2012 and 2013, respectively, for the treatment of adult patients with Philadelphia chromosome-positive chronic myelogenous leukemia with resistance or intolerance to prior therapy.
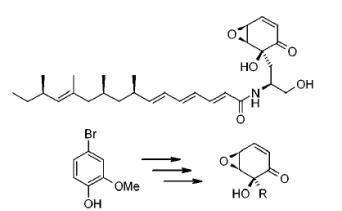
Runcie et al. reported [3] its application on the synthesis of novel scyphostatin analogues. Starting from commercially available 4-bromoguaiacol, oxidation using iodosobenzene diacetate in the presence of methanol gave rise to bromocyclohexa-2,4-dienone in excellent yield. The presence of the bromide atom does indeed reduce the propensity for dimerization, although some dimerization is observed on storage. Bromocyclohexa-2,4-dienone was immediately subjected to epoxidation under conditions to give the intermediate in an excellent 81% yield.
References
1.Surasani SR et al. Diels–Alder reaction of 4-halogenated masked o-benzoquinones with electron-rich dienophiles[J]. Tetrahedron Letters, 2011, 52:4615–4618
2.Zhu G. et al. New Synthesis of 7-(3-chloropropoxy)-4-hydroxy-6 -methoxyquinoline-3-carbonitrile, a Key Intermediate to Bosutinib[J]. Journal of Heterocyclic Chemistry, 2017, 2237-2241
Runcie KA, Taylor RJK. A Short and Efficient Route to Novel Scyphostatin Analogues[J]. Org. Lett., 2001, 3(21): 3237-3239