ChemicalBook > Articles Catagory List >Organic-reagents >what-is-diallyl-n-n-diisopropylphosphoramidite-
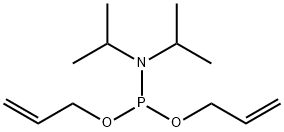
What is Diallyl n,n-diisopropylphosphoramidite?
Feb 11,2020
Diallyl N,N-diisopropylphosphoramidite was employed as a phophorylating reagent. It can be used for reactions involved in phosphitylation of alcohols, deallylation for the synthesis of nucleoside phosphoramidite, posphitylation and stereoselective pudovik rearrangement. It can be used for preparation of peptide substrates, rhodamine dyes with phosphorylated CH2OH sites, and 3-[4-(bis-allyloxy- phosphoryloxy)-2(R)-hydroxy-3,3-dimethyl-butyrylamino]-thiopropionic acid-S-propylester and so on. It also involved in the synthesis of pharmacologically active molecules including selective orally active S1P1 agonists, water-soluble prodrugs of triazole CS-758 with antifungal activity, and fostriecin analogs as antitumor agents, and so on.
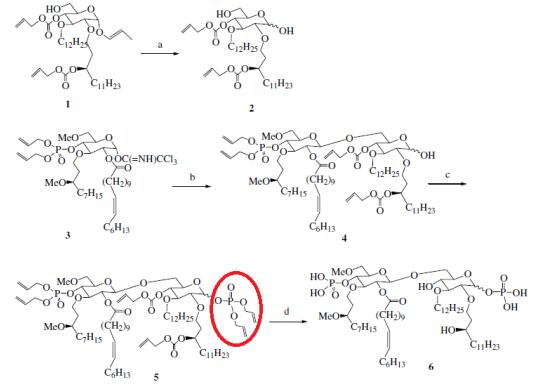

Shiozaki et al. reported [1] its application on the synthesis of glucose derivatives of E5564-related compounds. Reaction of 4 with diallyl diisopropylphosphoramidite in the presence of 1H-tetrazole, and successive oxidation of the resulting phosphite with hydrogen peroxide, afforded a 4:5 chromatographically inseparable mixture of a and b anomers 5. It was proved that the novel synthetic glucose–glucose disaccharide was effective toward blocking the agonistic effects of LPD in the human whole blood assay and the TNFa production in C3H/HeN mice. The compound was still active as the classic glucosamine–glucosamine type disaccharides.[1]
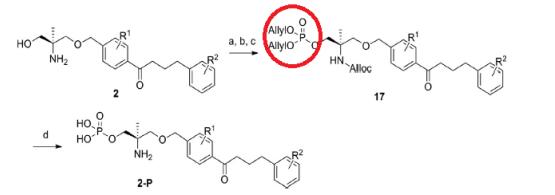
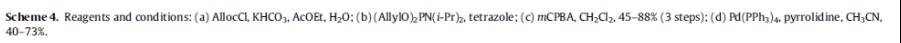
Tsuji et al. reported [2] its application on the synthesis of benzyl ether derivatives as potent orally active S1P1 agonists. After the protection of the amino group of 2 with allyl chloroformate (AllocCl), the primary hydroxyl group was phosphorylated by exposure with (AllylO)2PN(i-Pr)2 under the influence of tetrazole followed by an oxidation with mCPBA to provide 17 in good yield. Two allyl groups of a phosphate and an Alloc group were concurrently deprotected with pyrrolidine under the influence of a catalytic amount of Pd(PPh3)4 to give the phosphate of 2-P.
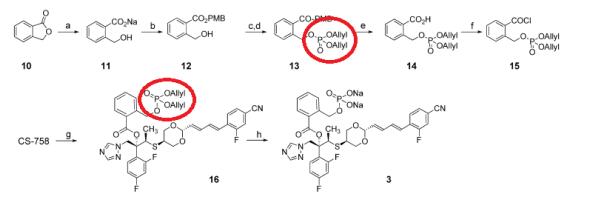

Kagoshima et al. reported [3] its application on the synthesis of antifungal activity of the novel water-soluble prodrug of antifungal triazole CS-758. The ester 12 was treated with diallyl diisopropylphosphoramidite in the presence of 1H-tetrazole and oxidized successively with tert-butyl hydroperoxide to give the corresponding phosphate 13. Compound 3 was converted to CS-758 rapidly and dephosphorylated intermediate alcohol was not observed in plasma. The conformational restriction had a distinct effect on the conversion rate of intermediate alcohols to drug CS-758.[3]
References
1.Shiozaki M. et al. Syntheses of glucose derivatives of E5564-related compounds and their LPS-antagonistic activities[J]. Carbohydrate Research, 2006, 341:811–822
2.Tsuji T. et al. Synthesis and SAR studies of benzyl ether derivatives as potent orally active S1P1 agonists[J]. Bioorganic & Medicinal Chemistry, 2014, 22:4246–4256