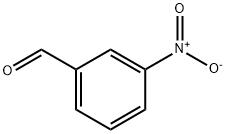
What is 3-Nitrobenzaldehyde?
Basic introduction
3-nitrobenzaldehyde belongs to the class of organic compounds known as nitrobenzaldehydes. These are nitrobenzenes that carry an aldehyde group at any position of the benzene ring. Based on a literature review a significant number of articles have been published on 3-nitrobenzaldehyde. This compound has been identified in human blood as reported by (PMID: 31557052 ). 3-nitrobenzaldehyde is not a naturally occurring metabolite and is only found in those individuals exposed to this compound or its derivatives. Technically 3-Nitrobenzaldehyde is part of the human exposome. The exposome can be defined as the collection of all the exposures of an individual in a lifetime and how those exposures relate to health. An individual's exposure begins before birth and includes insults from environmental and occupational sources.[1]

General procedure of 3-nitrobenzaldehyde
A mixture of benzyl halide (10 mmol) and H2O (20 ml) was stirred, and then the mixture was added to 4-hydroxypyridinium nitrate functionalized silica gel (2.46 g,11 mmol) and stirred. The reaction was done at ambient temperature for half the time indicated in Table 2 by microwave and then sodium hydroxide solution (0.440 g,11 mmol, in 10 ml H2O) was added dropwise over a period of 5 min at room temperature with vigorous stirring. The mixture was irradiated again for the other half time indicated in Table 2. The progress of the reaction was monitored by TLC (silica gel,n-hexane: pet. ether (1:3) as eluent). When the reaction was complete, the reaction mixture was cooled and extracted with diethyl ether (320 ml), washed with cold water, and dried over anhydrous sodium sulfate After filtration, the removal of solvent gave a crude product which was passed through a short silica gel column with n-hexane:diethyl ether (1: 1) as solvent to afford the pure product. The retrieved regent (4-hydroxypyridinesilica gel) was activated by treatment with 1.25 ml of concentrated HNO3 to provide 4-hydroxypyridinium nitrate SiO2, then reused for the oxidation.[2]
What is 3-Nitrobenzaldehyde used for?
3-Nitrobenzaldehyde is used as an intermediate for making dyes, photosensitive materials, and medicines like Nifedipine, Nimodipine, and Nicardipine. It's also a key ingredient in the production of pharmaceuticals, dyes, and surfactants. In the pharmaceutical industry, it's used to create a bunch of stuff including Iopanoic Acid Calcium, Iodofenac, Meta-Iodobenzyl Ester of Tartaric Acid, Nimodipine, Nicardipine, Nifedipine, and Niludipine. Plus, it's also used to make dyes, surfactants, and organic compounds for medicine. In pharma, it's involved in the production of Iopanoic Acid Calcium, Iodofenac, Cholecystographic Contrast Media, Meta-Hydroxy Tartaric Acid, and Diphenylmethylpyridine.[3]
Synthesis Catalyzed by 3-nitrobenzaldehyde
A wide variety of aromatic and aliphatic aldehydes underwent smooth reaction to give the corresponding acylals in good yield. As shown in, the results reveal that the BEA-SO3H catalysis generally results in good yields with aromatic aldehydes including those carrying electron donating or withdrawing substituents even when moderately hindered aldehydes were used, with the advantages of mild reaction conditions. The tolerance of various functional groups under the present reaction conditions is worthy of mention, where the acid sensitive or oxidizable groups such as methoxy, nitro, chloro, and double bonds do survive under such condition. Aldehydes with withdrawing substituents gave high although unmaximized yields, for example it is worth that o-nitrobenzaldehyde and 3-Nitrobenzaldehyde acetalised produced good yields (entries 7 and 14, 95% and 85%). Moreover, it should be noted that aliphatic aldehydes afforded the corresponding acylals within a short reaction time and good yield (entries 1 and 2). Compounds such as acetophenone (entry 3) and 2-butanone (entry 4) were not converted into the corresponding 1,1-diacetates at optimum conditions even for 30 min. Furthermore, the results reveal that BEA-SO3H shows good activity for the deprotection of 1,1-diacetates.
References
[1] Organic Preparations and Procedures International, 2021, vol. 53, # 2, p. 176 - 183]
[2] Phase Transitions, 96(9-10), pp.687-709.
[3] Journal of Organic Chemistry, 73(21), pp.8437-8447.
You may like
See also
Lastest Price from 3-Nitrobenzaldehyde manufacturers

US $0.00/KG2025-04-21
- CAS:
- 99-61-6
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $10.00/KG2025-04-21
- CAS:
- 99-61-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons