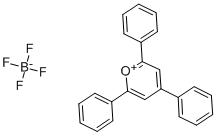
What is 2,4,6-triphenylpyrylium fluoroborate?
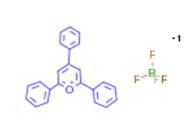
Fig 1. Chemical structure formula of 2,4,6-triphenylpyrylium fluoroborate
2,4,6-Triphenylpyrylium fluoroborate (C20H24ClN3O4, CAS registry No. 448-61-3) is a yellow crystalline powder. The melting point of 2,4,6-triphenylpyrylium fluoroborate is 250-257 oC. It is stable under recommended storage conditions. But it is moisture sensitive. So, it should store in cool place and keep container tightly closed in a dry and well-ventilated place. It can cause skin irritation, serious eye irritation and respiratory irritation.
2,4,6-Triphenylpyrylium fluoroborate can be used as photocatalyst to catalyzed the visible-light-induced intramolecular C-O bond formation. For example, an efficient photocatalytic system for the selective construction of benzyl cyclic ethers and lactones from alcohols and carboxylic acids was developed catalyzed by 2,4,6-triphenylpyrylium fluoroborate[1]. 2,4,6-Triphenylpyrylium fluoroborate enables the generation of benzylic radicals and subsequent intramolecular C-O cyclization through the intermediacy of benzyl alcohols. 2,4,6-Triphenylpyrylium fluoroborate can be used to catalyze photoinduced electron-transfer (PET)-promoted oxygenation reactions for the synthesis of benzo-fused 1,4-diaryl-2,3-dioxabicyclo[2.2.2]octanes, which are new antimalarial cyclic peroxides[2]. 2,4,6-Triphenylpyrylium fluoroborate can also readily catalyze PET reaction in acetonitrile to produce cyclopropenes and 2Hpyrroles from 3,3-dialkyl-4,5-diphenyl-3H-pyrazoles[3]. 2,4,6-Triphenylpyrylium fluoroborate can also be used as photocatalyst for the preparation of graphene oxide (GO)-based poly-heterozygosis pyridine nanomaterial (GO@PHPy), which not only have high mechanical strength, but also show excellent self-healing performance[4]. At the moderate contents of GO@PHPy, the GO@PHPy/PAA nanocomposite hydrogels (NC gels) exhibit the excellent mechanical properties, with tensile strength of 6.1 MPa, elongation at break of about 600%. And good self-healing properties are also witnessed on the healed NC gels, with tensile strength of 5.5 MPa and elongation at break 610%.
Due to the highly selective reaction between sulfides and 2,4,6-triphenylpyrylium fluoroborate, 2,4,6-triphenylpyrylium fluoroborate can be used as derivative reagent for the determination of sulfide with the high-performance liquid chromatography method based on pre-column derivatization[5]. After the reaction of sulfide ions with 2,4,6-triphenylpyrylium fluoroborate aiming at the formation of the corresponding thiopyrylium derivatives, they were separated on a C18 column using phosphate buffer and acetonitrile as eluent, and afterwards detected with a UV/vis detector.
References
[1] Im, H.; Kang, D.; Choi, S.; Shin, S.; Hong, S., Visible-Light-Induced C–O Bond Formation for the Construction of Five- and Six-Membered Cyclic Ethers and Lactones. Organic Letters 2018, 20 (23), 7437-7441.
[2] Kamata, M.; Hagiwara, J.-i.; Hokari, T.; Suzuki, C.; Fujino, R.; Kobayashi, S.; Kim, H.-S.; Wataya, Y., Applications of triphenylpyrylium salt-sensitized electron-transfer photo-oxygenation reactions to the synthesis of benzo-fused 1,4-diaryl-2,3-dioxabicyclo[2.2.2]octanes as new antimalarial cyclic peroxides. Research on Chemical Intermediates 2012, 39 (1), 127-137.
[3] Yen, Y.-P.; Huang, T.-M.; Tseng, Y.-P.; Lin, H.-Y.; Lai, C.-C., Photoinduced Electron Transfer Reactions of 3,3-Dialkylated 4,5-Diphenyl-3H-Pyrazoles: A New Route to the Formation of the Solvent Adducts. Journal of the Chinese Chemical Society 2004, 51 (2), 393-398.
[4] https://pubs.acs.org/doi/abs/10.1021/cr00028a009
[5] https://pubchem.ncbi.nlm.nih.gov/compound/9930615
See also
Lastest Price from 2,4,6-TRIPHENYLPYRYLIUM TETRAFLUOROBORATE manufacturers
US $2.20-8.80/kg2025-06-13
- CAS:
- 448-61-3
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 100kg

US $0.00-0.00/kg2025-03-03
- CAS:
- 448-61-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- kgs