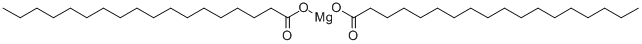Magnesium stearate-Physical and Chemical Properties
Magnesium stearate, alias: MAGNESIUM OCTADECANOATE; OCTADECANOIC ACID MAGNESIUM SALT; OCTADECONIC ACID MAGNESIUM SALT and STEARIC ACID MAGNESIUM SALT.molecular formula (C17H35COO) 2Mg. It is a white, non-sandy fine powder; and has a slight odor with a slippery feel when it comes in contact with the skin. Magnesium stearate is insoluble in water, ethanol or ether. It dissolves in hot water and hot ethanol, and decomposes into stearic acid and corresponding magnesium salt in case of acid. The structural formula is as follows.

Mainly used as lubricant, anti-adhesive, glidant. It is especially suitable for the granulation of oil and extract medicines, the granules made from it have good fluidity and compressibility. It can be used as a glidant in direct compression. It can also be used as a filter aid, clarifying agent and dripping agent, as well as a suspending agent and thickener for liquid preparations.
Ertel K D[1] et al. Characterized three batches of commercially available magnesium stearate based on their fatty acid composition, moisture content, and specific surface area. None of these variables appeared to have any effect on the lubricant activity of the samples. The lubricant properties of the compound were further examined using three hydrates of laboratory-prepared (pure) magnesium stearate. Based on the results obtained from the pure samples, it appears that differences in the lubricant properties of magnesium stearate are correlated with differences in moisture content and crystalline structure. The research by Leinonen U I[2] et al. Confirmed that the lubrication performance is related to the particle size distribution and specific surface area. The batch with a smaller particle size and larger specific surface area had considerably better lubricity.
The physicochemical and thermal properties of various forms of magnesium stearate were examined by Yasutaka Wada[3]et.al to establish their relationship with its lubricant abilities. The form of magnesium stearate having poor lubricating properties in commercial samples could be differentiated using differential scanning calorimetry (DSC). The lubricating properties were decreased in milled, dried and stored samples. These properties depend on the moisture content and the total enthalpy as seen from the DSC peaks corresponding to desorption of water, with the latter having a greater effect. The mechanism of lubrication seemed to involve water and/or gas molecules entering the spaces of the crystal lattice, causing a decrease in the interactive forces of the crystal lattice which, in turn, leads to easier shearing of the lubricant powder particles. Various researchers have conducted in-depth studies on the effects of solid state performance[4], mixing time, colloidal silica[5], temperature, and humidity on the lubrication efficiency of magnesium stearate[6].
References
[1] Ertel K D , Carstensen J T . Chemical, physical, and lubricant properties of magnesium stearate [J]. Journal of Pharmaceutical Sciences, 1988, 77(7):625-629.
[2] Leinonen U I , Jalonen H U , Vihervaara P A , et al. Physical and lubrication properties of magnesium stearate [J]. Journal of Pharmaceutical Sciences, 1992, 81(12):1194-1198.
[3] Wada Y . Pseudopolymorphism and lubricating properties of magnesium stearate[J]. Powder Technology, 1994, 78(2):109-114.
[4] https://pubs.acs.org/doi/10.1021/acs.oprd.6b00199
[5] https://pubchem.ncbi.nlm.nih.gov/compound/11177[6] Koivisto M , Jalonen H , Lehto V P . Effect of temperature and humidity on vegetable grade magnesium stearate[J]. Powder Technology, 2004, 147(1-3):79-85.
Related articles And Qustion
Lastest Price from Magnesium stearate manufacturers

US $0.00-0.00/kg2025-06-03
- CAS:
- 557-04-0
- Min. Order:
- 1kg
- Purity:
- 99.4%
- Supply Ability:
- 1000kg

US $0.00-0.00/kg2025-05-08
- CAS:
- 557-04-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20MT