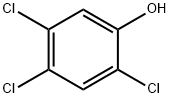
What is 2,4,5-Trichlorophenol?
General description
2,4,5-Trichlorophenol, with the CAS No: 95-95-4. This chemical’s molecular formula is C6H3Cl3O and molecular Weight is 197.45 g/mol. The boiling point is 246℃ at 760 mmHg. The melting point is 68℃ at 760 mmHg. The density is 1.678 g/cm3. The appearance is colorless needle-like crystals or gray flakes, with a strong odor of phenol.It should avoid contact with oxidants, acid anhydrides and acid chlorides. If swallowed or inhaled by mistake, it is highly toxic and has strong irritation. Poisoning can be absorbed through the skin. It can be used as a fungicide and gas chromatography comparison sample.

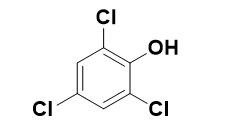
Figure 1 The structure of 2,4,5-Trichlorophenol
The synthesis of 2,4,5-Trichlorophenol
Method 1: Protected compound (1.0 equiv) was dissolved in MeOH/THF (1:1) in a sealed vial and stirred with thiophenolate resin 1 (1.45 mmol/g, 0.2 equiv). The reaction was heated as described herein. When reaction was complete, the resin was removed by filtration and washed with THF. The combined washings were dried in vacuo. If starting material remained, the product was isolated by standard column chromatography.
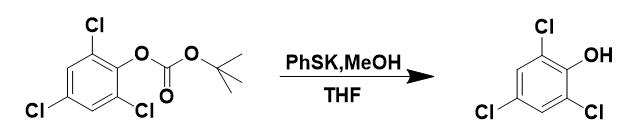
Figure 2 The synthesis of 2,4,5-Trichlorophenol
Method 2: (1)Chlorine reaction: 120 g of 2,4-dichlorofluoronitrobenzene and 600 mL of solvent acetic acid were added to the reaction vessel. Stir and control the temperature around 8 °C, and add NCS 97 g in batches. After the addition is complete, stir for a while and the GC is followed until the reaction is complete. After completion of the reaction, ice water was analyzed by water and suction filtered to obtain 133 g of 2,4,5-trichloronitrobenzene as an off-white solid. The content was more than 97%, and the yield was 94.3%.
(2)Reduction reaction: 130 g of iron powder was added to the reactor, the amount of water was adjusted, stirred, heated, and the temperature was controlled at 90 to 95℃ , and then 130 g of the above-mentioned nitro group was added in portions. After the addition, the GC tracks until the reaction is complete. After the reaction was completed, it was slightly cooled, extracted with ethyl acetate, filtered, filtered, and then evaporated to give a brown-brown solid, 2,4,5-trichloroaniline 89 g, content 98%, yield 79.1%.
(3)Phenol hydroxyl reaction on diazo: The raw material 2,4,5-trichloroaniline 85 g is dissolved in 720 g of 30% sulfuric acid solution, and a solution prepared from 42 g of sodium nitrite and 150 mL of water is added thereto with stirring. During the process, the reaction temperature is controlled at about 8 °C. After the addition is completed, the mixture is kept for a period of time to prepare a diazonium salt for use.
In another reaction flask, 56 g of copper sulfate pentahydrate and 200 g of a 30% sulfuric acid solution were added. Control the temperature at about 80 ° C, add the above diazonium salt, add, heat the reaction for a period of time, GC tracking until the reaction is complete. After completion of the reaction, the mixture was cooled, extracted with dichloromethane, and then evaporated to give a pale-yellow solid (yield: 63 g, content 97%, yield 73.7%).
Degradation of 2, 4, 5-trichlorophenol by the lignin-degrading basidiomycete Phanerochaete chrysosporium
Under secondary metabolic conditions the white rot basidiomycete Phanerochaete chrysosporium rapidly mineralizes 2,4,5-trichlorophenol. The pathway for degradation of 2,4,5-trichlorophenol was elucidated by the characterization of fungal metabolites and oxidation products generated by purified lignin peroxidase (LiP) and manganese peroxidase (MnP). The multistep pathway involves cycles of peroxidase-catalyzed oxidative dechlorination reactions followed by quinone reduction reactions to yield the key intermediate 1,2,4,5-tetrahydroxybenzene, which is presumably ring cleaved. In the first step of the pathway, 2,4,5-trichlorophenol is oxidized to 2,5-dichloro-1,4-benzoquinone by either MnP or Lip. 2,5-Dichloro-1,4-benzoquinone is then reduced to 2,5-dichloro-1,4-hydroquinone. The 2,5-dichloro-1,4-hydroquinone is oxidized by MnP to generate 5-chloro-4-hydroxy-1,2-benzoquinone. The orthoquinone is in turn reduced to 5-chloro-1,2,4-trihydroxybenzene. Finally, the 5-chlorotrihydroxybenzene undergoes another cycle of oxidative dechlorination and reduction reactions to generate 1,2,4,5-tetrahydroxybenzene. The latter is presumably ring cleaved, with subsequent degradation to CO2. In this pathway, the substrate is oxidatively dechlorinated by LiP or MnP in a reaction which produces a quinone. The quinone intermediate is recycled by a reduction reaction to regenerate an intermediate which is again a substrate for peroxidase-catalyzed oxidative dechlorination. This pathway apparently results in the removal of all three chlorine atoms before ring cleavage occurs.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Avoid direct sunlight. The package is sealed. It should be stored separately from oxidants, acid anhydrides, and acid chlorides, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with suitable materials to contain the leakage.
2. Lining material or iron drum outside the wooden box of the glass bottle. Store in a cool, ventilated warehouse; load and unload with care during transportation to prevent damage to the container.
Reference
[1]Michael, R, Pitts, et al. Indium metal as a reducing agent in organic synthesis[J]. Journal of the Chemical Society, 2001.
[2] Joshi D K , Gold M H . Degradation of 2,4,5-trichlorophenol by the lignin-degrading basidiomycete Phanerochaete chrysosporium.[J]. Appl Environ Microbiol, 1993, 59(6):1779-1785.
You may like
See also
Lastest Price from 2,4,5-Trichlorophenol manufacturers
US $0.00/kg2025-04-15
- CAS:
- 95-95-4
- Min. Order:
- 20kg
- Purity:
- 99%
- Supply Ability:
- 20 tons