2,5-Dichloroaniline - Pigment Uses, Synthesis etc.
General description
2,5-Dichloroaniline, with the CAS No: 95-82-9. This chemical’s molecular formula is C6H5Cl2N and molecular Weight is 162.02g/mol. The boiling point is 251℃ at 760 mmHg. The melting point is 47-50℃. The flash point is greater than 230 ℃. The density is 1.54g/cm3. The appearance is white needle crystal. It is easily soluble in ethanol, ether, carbon disulfide, but insoluble in water. It needs to be stored below +30°C. It is used as an intermediate for dyes and pigments, as well as for organic synthesis and the preparation of nitrogen fertilizer synergist. 2,5-dichloroaniline is an intermediate for the herbicide dicamba, as well as an intermediate for dyes and nitrogen fertilizer synergists.

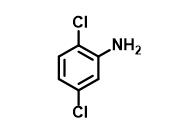
Figure 1 The structure of 2,5-dichloroaniline
The synthesis of 2,5-dichloroaniline
Method 1: The catalytic hydrogenation of 2,5-dichloronitrobenzene was performed in a Schlenk tube (25 mL). In a typical reaction, the catalyst (20 mg), 2,5-dichloronitrobenzene (1 mmol), and hydrazine hydrate (4 mmol) were sequentially added into a dried tube with ethanol (5 mL). Then, a thermostatic bath was connected to the tube for obtaining condensation, and the mixture was vigorously stirred at a reaction temperature of 60 °C.

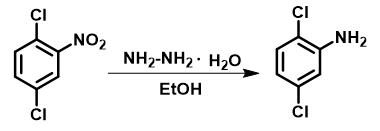
Figure 2 The synthesis of 2,5-dichloroaniline
Method 2: Add NH3·BH3 (3.0 eq) and CuO (5 mol%) successively to a solution of 2,5-dichloronitrobenzene (1 mmol) in methanol (6 mL) at 50 °C. Conduct the reaction without stirring to avoid the aggregation of CuO powder into hard, spherical particles with decreased specific surface area. Generate the hydrogen gas bubbles in situ during the reaction ensured the proper mixing of the reactants. Monitor the reaction progress by thin-layer chromatography. Recover solid CuO by centrifugation and wash with methanol to facilitate its use in additional reactions. Evaporate the reaction solution under reduced pressure to remove the solvent. Take the residue in water and extract with CH2Cl2 (or ethyl acetate). Collect the organic phase, dry over anhydrous magnesium sulfate and filter. Evaporate the filtrate under reduced pressure to obtain the crude product. Purify via silica gel chromatography (eluent: ethyl acetate/petroleum ether = 22/1) to obtain the product. 1H NMR (600 MHz, CDCl3): 7.141 (d, 1H, J = 8.4 Hz), 6.753 (d, 1H, J = 2.4 Hz), 6.647 (dd, 1H, J = 8.4, 2.4 Hz).13C NMR (100 MHz, CDCl3): δ 143.9, 133.1, 130.2, 118.8, 117.4, 115.4
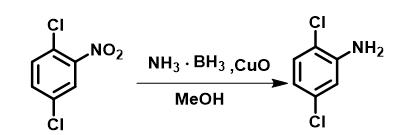
Figure 3 The synthesis of 2,5-dichloroaniline
The crystal structure of 2,5-dichloroaniline
The crystal structure of 2,5-dichloroaniline was previously determined from Weissenberg film data. The space group reported was P21/c and refinement converged at R = 0.126. The original cell dimensions were a = 13.237 (7), b = 3.892 (6), c = 18.80 (2) Å and β = 135.2 (2)°. The large β angle led to the examination of the cell dimensions with LEPAGE and the possibility of a tetragonal or orthorhombic cell was suggested. A new data set collected at 120 K refined to R = 0.026 in the space group P21/n with cell dimensions a = 13.1141 (7), b = 3.8137 (6), c = 13.1699 (7) Å and β = 90.033 (2)°. Final analysis with PLATON showed that the lattice featured metrical symmetry (pseudo-tetragonal or pseudo-orthorhombic) not supported by the cell contents which confirmed the crystal system as monoclinic. The P21/n designation is related to the size of the β angle, which is much closer to 90° in this setting, compared to the transformed cell. Taking the temperature of the determination into consideration, the cell dimensions originally reported and the cell dimensions transformed into P21/c from this study are equivalent; hence polymorphism is not shown. Details of the 2,5-dichloroaniline structure not previously reported include hydrogen-bond formation between amine groups that involves only one of the H atoms (H1B). This results in continuous chains of molecules running in the direction of the b axis that each contain two of the four symmetry-related molecules per unit cell required by the space group. The aromatic rings pack face-to-face to each other in these chains by translational symmetry along the b axis. The close separation of these rings (3.490 Å) indicates π–π-stacking interactions. There is also a short intramolecular H1B⋯Cl1 separation of 2.63 (2) Å but the N1—H1B⋯Cl1 angle is only 107 (2)°. The N atom deviates by 0.24 (1) Å from the plane defined by atoms C1, H1A and H1B. The shortest Cl⋯Cl separation is Cl1⋯Cl2(−½ + x, ½ − y, −½ + z) = 3.3219 (8) Å, compared to the previously reported value of 3.37 Å.The crystal structures of 2,3-, 2,4-, 2,6-, 3,4- and 3,5-dichloroaniline also show N—H⋯N hydrogen bonds and short Cl⋯Cl interactions.
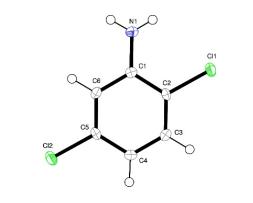
Figure 4 The molecular structure of 2,5-dichloroaniline
Reference
[1] Feng B , Xu Q , Wu X , et al. MOF-derived N-doped carbon composites embedded with Fe/Fe3C nanoparticles as highly chemoselective and stable catalysts for catalytic transfer hydrogenation of nitroarenes[J]. Applied Surface Science, 2021:149837.
[2] Feng B; Xu Q; Wu X; Ye, et al. Commercially Available CuO Catalyzed Hydrogenation of Nitroarenes Using Ammonia Borane as a Hydrogen Source[J]. ChemCatChem, 2020, 12(9).
[3] Cox P J. 2,5-Dichloro aniline, a monoclinic structure with a pseudo-tetragonal unit cell[J]. Acta Crystallographica Section E, 2001, 57(12).