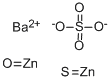Lithopone: Photo-Induced Zn2? Release, and Multianalytical Study of Historical Samples
Lithopone, brilliant white pigment used in paints, inks, leather, paper, linoleum, and face powder. It was developed in the 1870s as a substitute or supplement for lead carbonate (white lead), to overcome its drawbacks of toxicity, poor weathering, and darkening in atmospheres that contain sulfur compounds. Lithopone is an insoluble mixture of barium sulfate and zinc sulfide that precipitates upon mixing solutions of barium sulfide and zinc sulfate. The precipitate is recovered by filtration, then calcined (roasted) at temperatures above 600° C (1,112° F). Although it has been replaced in many applications by titanium dioxide, introduced after World War I, it is still widely used in a number of products, such as water paints. The composition of which underscores its superiority in specific applications. Ideally, prepared lithopone consists of 30 to 32 percent sulfide of zinc, and a negligible percentage of zinc oxide (1.5%), with the remaining majority being barium sulfate. These attributes render lithopone nearly comparable to the best grades of French process zinc oxide in terms of whiteness. Furthermore, its oil absorption, which sits between lead carbonate and zinc oxide, solidifies its position as a functional and efficient white pigment.

Significant zinc release from widely-used commercial lithopone pigments
The lithopone pigment, also referred to as Pigment White 5, is mainly comprised of zinc sulfide and barium sulfate. It is a commercial white pigment widely used in the production of paint, plastic, rubber, paper, enamel, and many other products, owing to its strong hiding power, good stability, and particularly the cheap production costs. Zinc is on the Priority Pollutant List regulated in Clean Water Act programs. The recommended level for zinc in ambient water is 5 mg/L based on the 1986 Quality Criteria for Water. We hypothesize that the solar exposure will lead to photoreactions of commercial lithopone pigments, resulting in the release of Zn2+ in natural aquatic systems. In this study, we aim to examine the photo-release of Zn2+ from commercial lithopone pigments under sunlight and discern the underlying mechanisms. The energy bands of the pigment were determined to understand its photochemical properties. The impacts of water chemistry on the photoreaction kinetics were examined for better assessing its environmental behavior. We also examined the photocurrent and surface photovoltage of the pigment to understand the reaction mechanisms. To the best of our knowledge, this is the first study that reported the significant photo-dissolution of commercial lithopone pigments and the consequent release of Zn2+ under environmental conditions.[1]
This study examined the photo-dissolution behavior of the commercial lithopone pigment and the subsequent Zn2+ release. The lithopone pigment readily underwent photo-dissolution under natural conditions, generating nanoparticles and releasing a significant amount of Zn2+ and SO42−. The commercial lithopone pigment tested is photoactive within the solar spectrum (wavelength <342 nm) with conduction band and valence band energies of −0.78 V and +2.85 V, respectively. Furthermore, it possesses good charge separation and transfer efficiency, generating O2•− and •OH. The photo-dissolution phenomenon was caused by the oxidation of the pigment lattice by photogenerated holes. The vast amount of lithopone pigments produced worldwide and their wide applications in industrial and consumer products will lead to their presence in the environment, especially urban systems. Our results suggest that a systematic assessment of the photochemistry and associated risks of lithopone pigments and the related products is in critical need. Furthermore, the photochemistry of other widely used inorganic pigments containing redox-active elements and possess good charge separation efficiency also needs to be examined for regulation purposes.
Multianalytical Study of Historical Luminescent Lithopone
Lithopone is a modern inorganic white pigment composed of a coprecipitate of zinc sulfide (ZnS) and barium sulfate (BaSO4). It was manufactured on a commercial scale starting in 1874 and sold under different names (Griffith white, Charlton White, Orr’s Zinc White). It found its application first in the cheaper grades of polish varnishes, floor paints, and paints for interiors as a substitute of lead white. Despite the cheapness of its manufacturing processes and good property as pigment, lithopone had the tendency to darken when exposed to sunlight. To prevent discoloration, starting in 1928, a small amount of cobalt, varying from 0.02% to 0.5% of the zinc content, was added prior to the calcination process. Nevertheless, due to the photodarkening effect, lithopone earned a bad reputation that made its usage as an artists’ pigment difficult to establish. In this work, we aim to provide new insights into the optical properties of historical lithopone samples through the combination of spectrally- and time-resolved PL imaging, for the quick identification and mapping of luminescent impurities, and electron paramagnetic resonance (EPR) analysis for the assessment of the presence of specific impurities and defects, which are responsible for the luminous properties of the pigment.[2]
The combination of spatial and temporal PL microscopic imaging enabled the identification and qualitative characterization of luminescent lithopone impurities, while EPR allowed a more complete description of the chemical composition of the material. The EPR results confirm the presence of CuZn2+ and MnZn2+ ions, acting as impurities in all the historical samples, whereas Ag traces could not be detected. Further, the EPR results indicate the presence of Fe as a constituent of the ZnS mineral ores. The multianalytical approach described in this work was designed to answer specific research questions about the optical properties of lithopone. In particular, in the historical lithopone samples studied, an intense PL emission is attributed to a synthesis process developed within a specific period and geographical area. The analysis of further historical samples of this pigment could help in understanding a possible correlation between these luminescence properties and sources and production methods. The results obtained encourage further exploration of the same protocol for the study of the intrinsic heterogeneity of other painting materials. Moreover, the information gained through this laboratory-based protocol can help in the future in the interpretation of results obtainable by means of in situ, nondestructive investigation of the luminescence properties of real paintings.
References
[1]Gao H, Yang S, Mao D, Long M, Qu X. Significant zinc release from widely-used commercial lithopone pigments under solar irradiation. Environ Pollut. 2022 Jan 1;292(Pt A):118352. doi: 10.1016/j.envpol.2021.118352. Epub 2021 Oct 9. PMID: 34637823.
[2]Bellei S, Nevin A, Cesaratto A, Capogrosso V, Vezin H, Tokarski C, Valentini G, Comelli D. Multianalytical Study of Historical Luminescent Lithopone for the Detection of Impurities and Trace Metal Ions. Anal Chem. 2015 Jun 16;87(12):6049-56. doi: 10.1021/acs.analchem.5b00560. Epub 2015 May 28. PMID: 26020448.
You may like
Lastest Price from Lithopone manufacturers

US $6.00/kg2025-04-21
- CAS:
- 1345-05-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $1000.00-400.00/kg2025-04-21
- CAS:
- 1345-05-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000