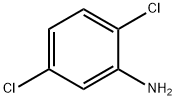
2,5-Dichloroaniline: Electrochemical Synthesis and Detection Method
2,5-Dichloroanilineit(2,5-DCA) is a colorless solid that is insoluble in water, it is an intermediate in synthesis of pesticides.

Electrochemical SynthesisMethod
2,5-dichloronitrobenzene was prepared from 2,5-dichlorobenzene by nitration with a mixture of sulfuric and nitri acids. The main substance content was 90%. Potassium phosphate, sulfuric acid, phosphoric acid, and other chemicals (pure or analytically pure grade) were used without additional purification.
Electrolysis was performed in the galvanostatic mode in a 150-ml temperature-controlled cell equipped with a reflux condenser, a ceramic diaphragm, and a platinum spiral anode. The cathode (several materials were tested) was treated with an acid solution and washed with a large amount of water before the experiment. Reduction in an aqueous-ethanolic solution of sulfuric acid was performed as follows. The cathode compartment (with a lead cylinder as cathode) was charged with 100 ml of a solution containing 7.5 wt % sulfuric acid and 50 wt % ethanol. Then 7.9 g (0.0402 mol) of 2,5-dichloronitrobenzene was added. The anolyte was 25% aqueous H2SO4.
The mixture was heated to 50℃, and reduction with a current of 6.5 A (cathodic current density 750 A m2) was performed for 1 h 29 min with vigorous stirring. After electrolysis completion, the mixture was unloaded and kept for 30 40 min at 35℃. The precipitate of 2,5-DCA sulfate was filtered off and dried at room temperature; 9.2 g of 2,5-DCA sulfate was obtained. The product contained about 62.8% DCA and 34% sulfate ions. Yield based on 2,5-dichloronitrobenzene 88.7%, current efficiency 59.1%. Reduction in the two-phase system was performed with a cadmium cathode (working surface area 87 cm2). The cathodic compartment was charged with 70 ml of a 1 N potassium phosphate solution (pH 5), 10 g (0.052 mol) of 2,5-dichloronitrobenzene, and 30 ml of toluen.
The anolyte was 20% aqueous H3PO4. Electrolysis was performed with a current of 6 A (cathodic current density 700 A m2 ) for 1 h 20 min and then with a current of 3 A for an additional 1 h 20 min. In the course of the electrolysis, the mixture in the cathodic compartment was vigorously stirred; pH 5.0 was adjusted at regular intervals by dropwise addition of phosphoric acid. The temperature was kept in the range 50-52℃. Yield of 2,5-DCA 7.3 g (main substance content 92%, yield based on 2,5-dichloronitrobenzene 80%, current efficiency 56.
Reference
[1] V. R. Islamgulova.“Electrochemical Synthesis of 2,5-Dichloroaniline.”Russian Journal of Applied Chemistry 76 7 (2003): 1076–1078.