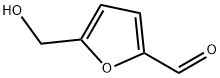
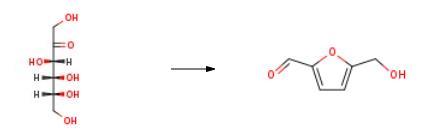
What are the side effects of 5-Hydroxymethylfurfural?
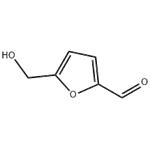
5-Hydroxymethylfurfural (5-HMF), which is derived from sugars, is the link between biomass and furan-based chemicals. With its various functional groups and associated reaction sites, 5-HMF opens the door to various chemical modifications, making it a versatile, renewable building block.
5-Hydroxymethylfurfural has been included among the top 10 most valuable biobased products from biorefinery of carbohydrates by the US Department of Energy in 2010[1]. It serves as a platform for the production of an array of fuel and chemical products such as dimethylfuran (DMF), 2,5-furandicarboxylic acid (FDCA), levulinic acid, 2,5-diformylfuran (DFF), 5-hydroxymethyl-5-furan carboxylic acid (HMFCA), dihydroxymethylfuran, 5-formyl-2-furan carboxylic acid (FFCA), 1,6-hexanediol, adipic acid, etc. The production of 5-HMF has thus attracted enormous attention, which is evident by many publications, including several reviews that have appeared regularly since the early years of the 21st century, although the compound has been known since the end of the 19th century.
During thermal processes, compounds such as furan, methyl furans, acrylamide and 5-hydroxymethylfurfural (5-HMF) are formed. Indeed, 5-HMF, a heat-induced food toxicant, is formed in many food items and causes several adverse effects in animals.
The mechanisms of 5-HMF formation can be divided into two main pathways. The first involves the decomposition of 3-deoxyglucosone (3-DG)(the Maillard reaction), and the second involves the dehydration of sugars under acidic conditions (caramelization)[2].
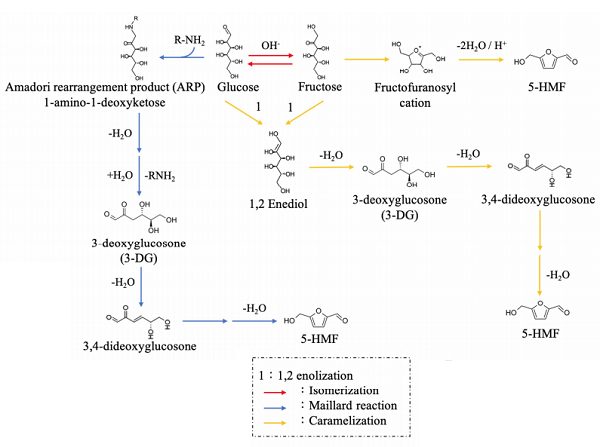
Maillard reaction: The Maillard reaction is a non-enzymatic browning reaction occurring widely in processed foods. In heat, the reaction begins with reducing sugars (e.g., glucose, fructose) and amino acids, forming a Schiff base. Subsequently, Schiff base cyclization results in the formation of Heyns rearrangement products and Amadori rearrangement products. As mentioned above, these are unstable, and they mainly degrade to 1,2-eneaminnol by 1,2-enolization at pH values ≤7.1,2-enolization is the rate-determining step in 5-HMF formation. Afterwards, dehydration of 1,2-eneaminnol results in the generation of 3-DG, regarded as the key intermediate in HMF formation. Finally, 3-DG forms 3,4-dideoxyglucosone (3,4-DGE) by dehydration yielding 5-HMF through cyclization.
Caramelization: Sugars exposed to acidic conditions during thermal treatments can also give rise to 5-HMF. Acid-catalyzed degradation of fructose forms the fructofuranosyl cation initially, and the cation can be converted into 5-HMF, especially in dry systems. Additionally, glucose and fructose can form 3-DG through 1,2-enolization and dehydration, after which dehydration and cyclization yield 5-HMF.
Exposure to 5-HMF is inevitable for human beings since it is widespread in foods, including honey, bread, beer, coffee, fruit juices, and black garlic. Many studies have shown that 5-HMF has diverse effects on human health, including carcinogenicity, neoplastic transformation, hepato- and nephrotoxicity, and antioxidant activity[3]. The toxicological impact of HMF have been demonstrated in several experimental animals and cultured mammalian cells. The daily human intake of HMF was reviewed by Husøy et al. and ranged from 30 to 150 mg/person/day, a dose much higher than that of other food heat-borne toxicants like furan and acrylamide. The Federal Institute of Risk Assessments estimated a range from 4 to 30 mg HMF/person/day.
Studies to date have used a variety of food processing technologies to mitigate 5-HMF formation or remove 5-HMF in foods, including UV irradiation, yeast fermentation, the addition of flavan-3-ol-like compounds, vacuum treatment, and microwave heating.
References
[1] Sayed, Mahmoud et al. “5-Hydroxymethylfurfural from fructose: an efficient continuous process in a water-dimethyl carbonate biphasic system with high yield product recovery†.” Green Chemistry 16 (2020): 5402–5413.
[2] Chieh-Hsiu Lee . “Recent advances in processing technology to reduce 5-hydroxymethylfurfural in foods.” Trends in Food Science & Technology 93 (2019): Pages 271-280.
[3] Mayada R Farag. “The Toxicological Aspects of the Heat-Borne Toxicant 5-Hydroxymethylfurfural in Animals: A Review.” Molecules 25 8 (2020).
You may like
Related articles And Qustion
Lastest Price from 5-Hydroxymethylfurfural manufacturers
US $0.00/kg2025-10-22
- CAS:
- 67-47-0
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $10.00/kg2025-04-21
- CAS:
- 67-47-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt