What are the effects of fumagillin on bees?
Introduction
Fumagillin is one of the mycotoxins. First isolated from A. fumigatus in 1949, it is encoded inside a supercluster on chromosome eight. The target of this mycotoxin is the methionine aminopeptidase (MetAP) type 2 enzyme, to which it binds and inactivates irreversibly. Fumagillin is a potent fungal metabolite. It is widely used in apiculture and human medicine against various microsporidian fungal infections. It has been the subject of research in cancer treatments by employing its angiogenesis inhibitory properties.

Chemical property
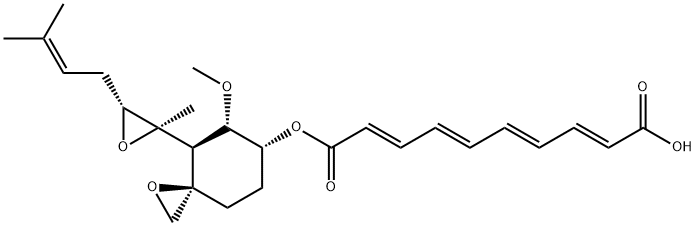
Fumagillin is a small molecule with a molecular weight of 458.54 g·mol−1. A decatetraenedioic acid connected to cyclohexane by an ester bond characterizes its chemical structure. The cyclohexane also has a methoxy group, an epoxide, and an aliphatic chain that derives from a terpene and contains another epoxide. These epoxides are, in part, responsible for the instability of the molecule. As a relatively non-polar molecule (predicted partition coefficient, logP: 4.05), fumagillin has poor water solubility (3.3 mg·L−1) but can be dissolved in organic solvents such as ethanol or DMSO. However, owing to the acidic nature of the carboxylic group (predicted pKa: 4.65), the molecule's polarity increases with pH (predicted distribution coefficient, logD: 0.53 at pH 10). Therefore, the solubility of fumagillin in water is highly dependent on pH[1].
Biological activity
Fumagillin inhibits the enzyme methionine aminopeptidase-2 (MetAP2) and is known to block MetAP2 in Encephalitozoon cuniculi, a microsporidian pathogen of humans. Most eukaryotes possess genes for two MetAP isoforms, MetAP1 and MetAP2, and require either MetAP1 or MetAP2 to survive[2]. As MetAPs are essential for the hydrolyzation of the initial methione (iMet) located in the N-terminal of the new proteins being synthesized, any imbalance produced by MetAP2 inhibition can affect many proteins, some of them implicated in the correct maintenance of cellular safety. This activity is the basis of the different effects associated with fumagillin. On the one hand, this toxin showed an antibiotic effect as amoebicidal activity inhibiting the growth of Entamoeba histolytica and shows similar functions during interaction with macrophages. Besides, fumagillin has pharmaceutical potential for treating microsporidiosis, as it is the only effective chemical treatment currently available for nosemiasis caused by the parasitic fungi from the Microsporidia phylum on Apis spp. It is usually used for the treatment of pests in bee hives.
Toxicity
Fumagillin is the only antibiotic approved for controlling nosema disease in honey bees. It has been extensively used in United States apiculture for over 50 years to control nosema apis, which is a unicellular parasite spread by spores that infect honey bees. Bees develop extreme diarrhea frequently, resulting in death and a decrease in a hive's honey production. Fumagillin is toxic to mammals and must be applied seasonally and cautiously to avoid honey residues. Fumagillin degrades or is diluted in hives over the foraging season, exposing bees and the microsporidia to declining drug concentrations [2]. Spore production by Nosema ceranae, an emerging microsporidian pathogen in honey bees, increased in response to declining fumagillin concentrations, up to 100% higher than that of infected bees that have not been exposed to fumagillin. N. apis spore production was also higher, although not significantly so.

Due to the toxicity of fumagillin, it should be used very carefully, and it cannot be used widely. Therefore, less toxic derivatives have been developed to replace fumagillin in some applications. On the other hand, fumagillin has antiangiogenic activity, probably because of its inhibitory activity against the MetAP2 enzyme; consequently, it has valuable pharmaceutical potential and a potential role in cancer treatment [3]. Moreover, this toxin is able to inhibit the function of neutrophils, inducing cell death in erythrocytes. It plays a role in damaging lung epithelial cells, which opens the way to fungal invasion, perhaps owing to its antiangiogenic properties.
References
[1] Xabier Guruceaga. “Fumagillin, a Mycotoxin of Aspergillus fumigatus: Biosynthesis, Biological Activities, Detection, and Applications.” Toxins 12 1 (2019).
[2] Wei-Fone Huang. “Nosema ceranae escapes fumagillin control in honey bees.” PLoS Pathogens 9 3 (2013): e1003185.
[3] Johan P. van den Heever*. “Fumagillin: An Overview of Recent Scientific Advances and Their Significance for Apiculture.” Journal of Agricultural and Food Chemistry 62 13 (2014): 2728–2737.
You may like
See also
Lastest Price from Fumagillin manufacturers

US $1.00-4.00/KG2025-05-14
- CAS:
- 23110-15-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $1.00/g2025-02-13
- CAS:
- 23110-15-8
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 10000