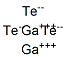Gallium telluride:Crystal structure,Synthesis,Toxicity
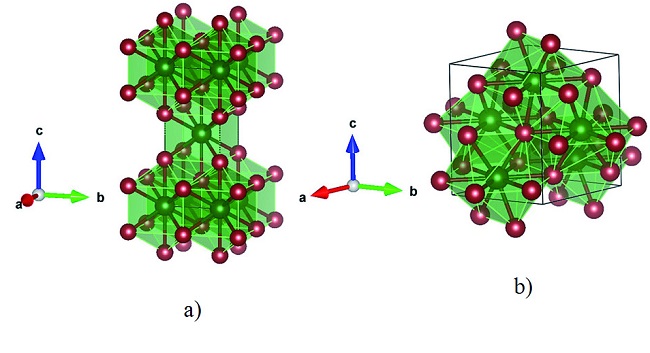
Gallium telluride is an odorless, black, brittle crystalline solid and is a semiconductor of the III-VI type that crystallizes in a lattice structure.
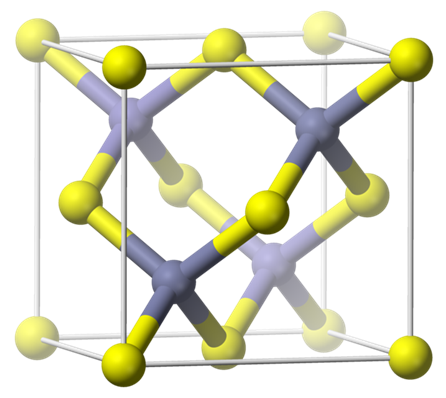
Crystal structure
Cubic, Sphalerite Structure - Space Group F(-4)3m
Chemical properties
Gallium telluride is stable at room temperature. The compound is relatively unreactive, and there are no known materials with which it is incompatible. Gallium telluride will over time emit telluride fumes and it naturally decomposes. There is no risk of hazardous polymerization.
Synthesis
Gallium telluride is most commonly synthesized through the solid-state reaction of trimethylgallium and a telluride oxide complex under high temperatures. It is also possible to synthesize the compound by reacting elemental gallium and elemental tellurium at high temperatures.
Toxicity
Gallium telluride is converted in the body to dimethyl telluride which imparts a garlic-like odor to the breath and sweat. In addition, heavy exposures may result in headache, drowsiness, metallic taste, loss of appetite, nausea, tremors, convulsions, and respiratory arrest.