Tungsten Silicide Crystal Structure
Tungsten based silicides, and in particular tungsten disilicide (WSi2) with melting point above 2160 °C and pentatungsten trisilicide (W5Si3) with melting point above 2320 °C, respectively, appear suitable for operation in extreme conditions. In addition, WSi2 has low electrical resistivity and good thermal stability, and it has been investigated as a protective coating on W-based alloys because of its excellent oxidation resistance.
Tungsten silicidc is one of numerous important refractory compounclscurrcntly being studied experimentally for utilization as a high temperature material in the space program. Tungsten disilicide (WSi2) was synthesized by simple thermal treatment at 1350 °C for 4 h in an argon atmosphere. These optimal synthesis conditions were obtained by variation of temperatures and times of heating, and the structure of the final synthesized compound was determined by XRPD analysis.
New Crystal Structures
Tungsten Silicide (WSi2) crystallizes in the tetragonal space group I4/mmm (no. 139). Furthermore, the equilibrium WSi2 tetragonal modification is characterized as the MoSi2 structure type (Strukturbericht designation C11b, and Pearson symbol tI6). The MoSi2 crystal structure is characteristic of the sequential stacking of the W and Si atomic layers along the c axis, with ten-fold coordination of tungsten by Si atoms (CN = 10, see Figure 1a).
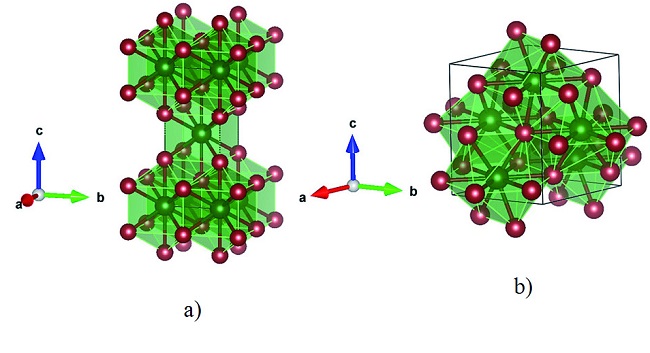
Another experimentally observed structure of WSi2 at low temperatures and/or in thin films shows hexagonal symmetry with space group P6222 (no. 180). In the literature, the hexagonal modification of tungsten disilicide has been defined as metastable with CrSi2 type of structure (Strukturbericht designation C40, and Pearson symbol hP9). The hexagonal WSi2 modification keeps the ten-fold coordination of tungsten by Si atoms, but changes the coordination polyhedron (see Figure 1b).