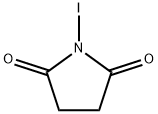
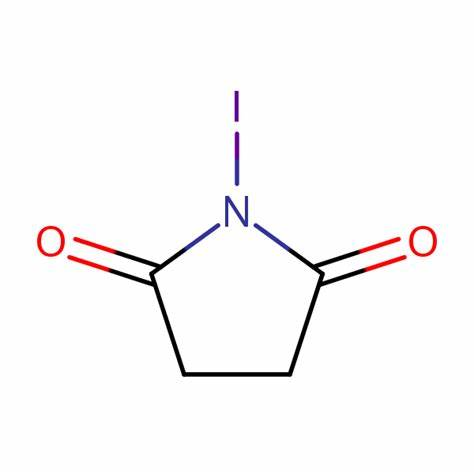
What are the chemical reactions involved in N-Iodosuccinimide?
N-Iodosuccinimide (NIS) is an electrophilic iodide. The use of NIS as an electrophilic iodinating agent for aldehydes, ketones and alkenes is well reported but recent developments have seen a host of further applications, such as the use of Brønsted or Lewis acids to improve its reactivity in electrophilic aromatic substitution reactions, asymmetric iodination of aldehydes using an axially chiral catalyst and 1,3-dicarbonyl compounds under mild conditions, synthesis of haloalkenes and haloalkynes by catalytic Hunsdiecker reaction, the reaction of alkynes and NIS/water to give α-diketones, highly enantioselective iodocyclization of polyprenoids and Au-catalyzed formation of 2-iodoenones from propargylic alcohol derivatives, amongst others.
Interestingly, there have also been a few reports on the use of NIS as a mild and selective Lewis acid, such as in the catalytic deprotection of TBDMS ethers to alcohols in methanol under ambient conditions and the N-debenzylation of benzylamino alcohols. Cyclodehydration of Bohlmann-Rahtz aminodienone intermediates using N-iodosuccinimide as a Lewis acid proceeds at low temperature under very mild conditions to give the corresponding 2,3,6-trisubstituted pyridines in high yield and with total regiocontrol.
References:
[1] MARK C BAGLEY; Christian G. Bohlmann-Rahtz cyclodehydration of aminodienones to pyridines using N-iodosuccinimide.[J]. Molecules, 2010. DOI:10.3390/molecules15053211.
Related articles And Qustion
See also
Lastest Price from N-Iodosuccinimide manufacturers

US $9.90/KG2025-04-21
- CAS:
- 516-12-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $6.00/kg2025-04-21
- CAS:
- 516-12-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month