Voriconazole:azole drug
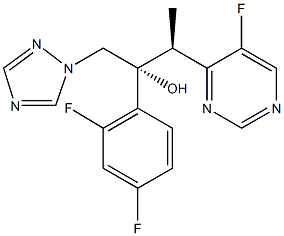
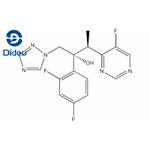
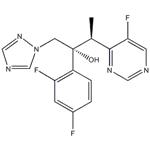
Voriconazole (Vfend, UK-109,496) is a synthetic triazole derivative with potent broad-spectrum activity. Its structure is similar to fluconazole, but one triazole ring is replaced with a fluorinated pyrimidine and an a-methyl group is added to the propanol backbone. Available formulations include a powder complexed with sulfobutylether b-cyclodextrin (SEBCD) for intravenous administration and two oral preparations, film-coated tablets and a powder for oral suspension, that do not contain SEBCD. Like other azoles in its class, voriconazole acts by inhibiting 14a-lanosterol demethylation, a critical step in fungal ergosterol biosynthesis. It demonstrates activity against a wide range of yeast and filamentous fungi, including Candida, Cryptococcus, Aspergillus, and dimorphic fungi. Voriconazole is currently approved by the US Food and Drug Administration and the European Medicines Agency for the primary treatment of invasive aspergillosis, mucosal and systemic candidiasis, and refractory fungal infections caused by Scedosporium spp. or Fusarium spp.
Mechanism of Drug Action
Like the other azole drugs, the primary mode of action of voriconazole is inhibition of fungal cytochrome P450-dependent C14-a sterol demethylase. Inhibition of this key step in ergosterol biosynthesis results in accumulation of lanosterol and depletion of ergosterol. These plasma membrane changes increase membrane permeability and halt fungal growth.
Drug interactions
Voriconazole is both a substrate and an inhibitor of the CYP450 enzymes, specifically CYP2C19, CYP2C9, and CYP3A4. Therefore, a patient’s medications should be reviewed for potential drug interactions. Voriconazole is a more potent inhibitor of CYP3A4 than fluconazole, but is not as potent as itraconazole. Co-administration of voriconazole with astemizole, cisapride, pimozide, quinidine, or terfenadine is contraindicated because of the risk of QT prolongation and torsades de pointes. Inducers of CYP450, including long-acting barbiturates, carbamazepine, rifampicin, rifabutin, and high-dose ritonavir (400 mg twice a day), may result in subtherapeutic voriconazole serum levels and are contraindicated. Voriconazole can substantially increase serum levels of ergot alkaloids and concomitant use is also contraindicated.
Concomitant administration of calcineurin inhibitors and voriconazole inhibits metabolism of calcineurin inhibitors via CYP3A4. In renal transplant patients receiving cyclosporine, co-administration of voriconazole increased exposure to cyclosporine 1.7-fold. Following initiation of voriconazole, it is recommended that cyclosporine levels be halved and levels be monitored closely. In addition, tacrolimus exposure has been shown to increase more than 3-fold when administered with voriconazole. Sirolimus and voriconazole are expected to have a particularly significant interaction requiring a 90% dosage reduction of sirolimus. Voriconazole alters metabolism of midazolam, resulting in a 31–84% decrease in clearance of midazolam. If used concomitantly, the midazolam dosage should be reduced substantially, although the combination is not recommended.
Related articles And Qustion
See also
Lastest Price from Voriconazole manufacturers
US $0.00-0.00/KG2025-12-01
- CAS:
- 137234-62-9
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS
US $0.00/kg2025-11-21
- CAS:
- 137234-62-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise