Is Voriconazole safety?
Voriconazole is a broad‐spectrum triazole active in vitro against various yeasts and moulds, including Aspergillus species. It is a derivative of fluconazole but has a broader spectrum, and it can be given orally and intravenously.

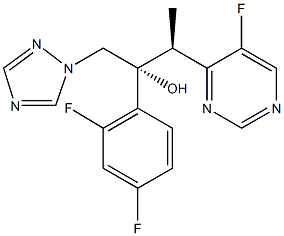
Voriconazole was approved by the US Food and Drug Administration (FDA) in May 2002. It is structurally derived from fluconazole, with 1 triazole moiety replaced by a fluoropyrimidine group and a methyl group added to the propanol backbone. Voriconazole is indicated for treating invasive aspergillosis and serious fungal infections caused by Scedosporium apiospermum and Fusarium species in patients who cannot tolerate or are refractory to other therapies. Chemically, voriconazole is (ZR,3S)-2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-l-(lH-1,2,4- triazol-1-yl)-2-butanol, with the molecular formula C16H14F3N5O and a molecular weight of 349.3 kd.
Some 2090 patients have been exposed to voriconazole in clinical or pharmacologic trials. Five hundred fifty-eight patients received 212 weeks of voriconazole therapy, and 38 received 21 years of therapy. During these studies, therapy was discontinued in 16.2% (339/2090) of patients. The chief reasons for discontinuation of voriconazole therapy in the pooled data from these early studies were elevations in alkaline phosphatase (1.4% [29/2090)]), acute kidney failure (1.3% [28/2090]), liver function test results ~3 times ULN (1.1% [23/2090]), hepatic enzymes ~3 times ULN (1.0% [20/2090]), rash (1.0% [20/2090]), sepsis (1.0% [20/2090]), fungal infection (0.9% [18/2090]), respiratory disorders (0.8% [17/2090]), bilirubin >1.5 times ULN (0.7% [14/20901), abnormal vision (0.5% [11/2090]), cholestatic jaundice (0.5% [10/20901), fever (0.5% [10/2090]), and hallucinations (0.4% [8/2090]).
The results of in vitro susceptibility studies and case reports suggested that voriconazole may be helpful to against fluconazole- and/or itraconazole-resistant strains of Candida. Although voriconazole may be associated with a lower incidence of serious systemic adverse effects compared with amphotericin B (13.4% vs 24.3% in 1 pivotal clinical study; P = NS), significant adverse effects associated with voriconazole include visual abnormalities ( approximately 30%), skin reactions ( approximately 20%), and elevations in hepatic enzymes (< or =20%). Voriconazole is available in oral and intravenous formulations. Pharmacokinetically, it has a widespread distribution, including penetration into cerebral tissue. However, as 80% of voriconazole is hepatically eliminated, primarily via the cytochrome P450 (CYP) isozymes CYP2C19, CYP3A4, and CYP2C9, voriconazole has a high potential for drug interactions, and dose reduction is recommended in patients with mild to moderate hepatic dysfunction (Child-Pugh class A or B). Oral voriconazole may be preferred in patients with a creatinine clearance <50 mL/min due to the potential accumulation of the solubilizing excipient in the parenteral formulation of voriconazole.
Related articles And Qustion
See also
Lastest Price from Voriconazole manufacturers
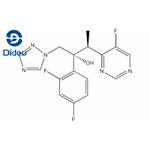
US $0.00-0.00/KG2025-12-01
- CAS:
- 137234-62-9
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS
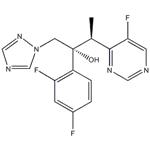
US $0.00/kg2025-11-21
- CAS:
- 137234-62-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise