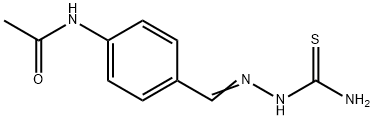
Mechanism of action of Thiacetazone
Thiacetazone (also called thioacetazone or amithiozone) was synthesized by Behnisch and Schmidt and investigated clinically by Gerhard Domagk and co-workers in Germany in the 1940s. It was subsequently shown to be effective alone or when combined with streptomycin in patients with tuberculosis in the United States. It is active against Mycobacterium tuberculosis, but was initially considered too toxic for widespread use. However, as it is extremely cheap to manufacture and stable in harsh climatic conditions, thiacetazone became a common component of tuberculosis treatment protocols in many developing countries. In patients co-infected with human immunodeficiency virus (HIV), thiacetazone causes high rates of severe cutaneous reactions, leading to controversy about its continued role in the treatment of tuberculosis in this setting. Thiacetazone has little role in the treatment of multidrug-resistant tuberculosis, except as a last resort.
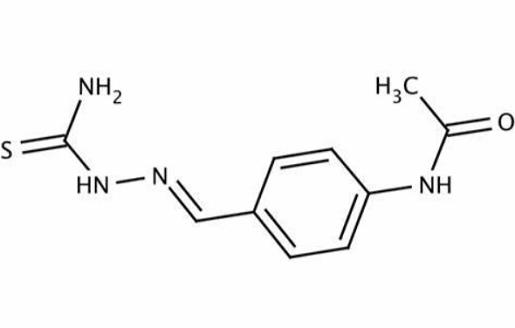
Mechanism of action
Thiacetazone is a prodrug that requires activation by a bacterially encoded monoxygenase, EthA, to be active. EthA also activates ethionamide, and it would be expected that mutations in the corresponding gene, etha, would result in complete cross-resistance. This is well illustrated in a study of MDR-TB isolates obtained from Cape Town: 11 of 11 ethionamide-resistant isolates that had mutations in etha were also resistant to thioacetazone.
The target of activated thiacetazone has been elusive and its mechanism of action poorly understood, although it is thought to interfere with mycolic acid synthesis. Mycolic acids are complex long-chain fatty acids, and the molar ratios of different mycolic acids influence fluidity and permeability of the cell wall and the immunologic response of the host. Part of the variability in mycolic acid structure results from the actions of cyclopropane mycolic acid synthases (CMASs) that convert double bonds in mycolic acid intermediates to cyclopropane residues.
Laboratory studies with M. marinum and M. bovis-bacille Calmette– Gue´rin (BCG) have recently shown that activated thiacetazone probably binds to and inhibits certain CMASs, leading to alteration of the cyclopropanation status of the cell envelope that is deleterious to cell function. The inhibitory effect of thiacetazone in these experiments was reversed by overexpressing cmaA2 and related genes. Interestingly, a null mutant of cmaA2 in M. tuberculosis was hypervirulent, suggesting, at least in theory, a basis for the paradoxical effects of thiacetazone that have been observed in in vitro studies.
Bioavailability
Thiacetazone is well absorbed following oral administration, with only minimal amounts appearing unchanged in feces. The serum half-life of the drug is approximately 12–16 hours.
Toxicity
The most important toxicity of thiacetazone is skin rash. In the pre- HIV era, this side-effect occurred in approximately 10% of patients. However, the risk appeared to be partly related to race as cutaneous reactions were less common in Africa than in East Asia, where thiacetazone was considered too toxic to use. Most cases were mild, maculopapular in nature, appeared during the first 6 weeks, and resolved despite continued treatment. However, about 20% were severe and occasionally caused fatal erythema multiforme, toxic epidermal necrolysis, or Stevens– Johnson syndrome.
Gastrointestinal side-effects
Nausea, vomiting, anorexia, diarrhea, and indigestion are common and troublesome, and can be reduced by taking the tablets with food. Anorexia may be sufficient to cause weight loss. Up to 25% of patients may have to discontinue treatment owing to gastrointestinal intolerance. Hepatitis is relatively common, including when thiacetazone is used without isoniazid.