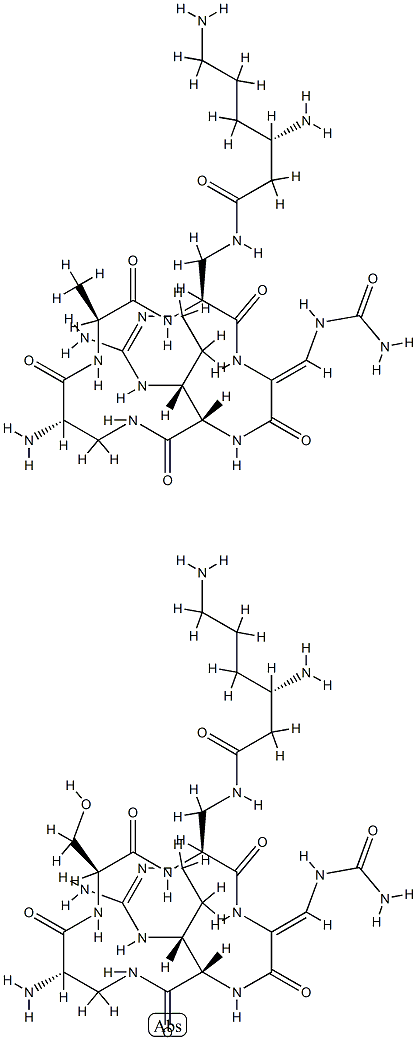
Mechanism of action of Capreomycin
Capreomycin is an important reserve antibiotic for the treatment of tuberculosis and has been used for this purpose for more than 40 years. It is a naturally produced cyclic polypeptide first isolated in 1959 from Streptomyces capreolus at the Lilly Laboratories in Indiana. Capreomycin consists of four microbiologically active compounds – capreomycin IA, IB, IIA, and IIB – the complete structure of all of which has not been fully elucidated. Capreomycin is often grouped together with the aminoglycosides as they share similar pharmacokinetics and toxicities, but the spectrum of activity of capreomycin is restricted to mycobacteria. For clinical purposes capreomycin is given as the sulfate.

Mechanism of action
The mode of action of capreomycin has not been fully elucidated, but it inhibits bacterial protein synthesis by interacting with the bacterial ribosome in a manner analogous to the aminoglycosides. However, there may also be other targets, and capreomycin appears to have a unique action against stationary-phase mycobacteria.
Capreomycin binding to the 16S ribosomal subunit has recently been shown to require active methylation of two specific ribosomal sites by a bacterially encoded methylase. The inactivation of this methylase gene, tlyA, is one mechanism of acquiring capreomycin resistance, and the absence of tlyA analogs in most nonmycobacterial pathogens may explain why the activity of capreomycin is restricted to mycobacteria.
Use
Capreomycin is a key drug in the treatment of MDR-TB in combination with other second-line drugs to which sensitivity has been demonstrated.
Bioavailability
Absorption of capreomycin from the gastrointestinal tract is minimal and it must be given parenterally (i.m or i.v.). There has been recent interest in developing aerosol delivery systems, but to date there are no reports of success using this route of delivery in human tuberculosis. Peak concentration occurs approximately 1–2 hours after an i.m. injection; the elimination half-life in patients with normal renal function is 4–6 hours.
Concentration–time curves are similar with i.m. or i.v. administration, but peak serum levels are 30% higher with i.v. administration. Serum levels are low at 24 hours and accumulation is not observed at 30 days in individuals with normal renal function. After a single 1-g intravenous infusion of capreomycin in seven healthy adults, clearance of capreomycin was 5.7371.54 l/kg/h; capreomycin clearance correlated closely with creatinine clearance, and reduced proportionately in patients with renal impairment. Capreomycin clearance is significantly increased with hemodialysis. By analogy with the aminoglycosides, capreomycin is likely to have reduced toxicity and preserved efficacy with intermittent dosing.
Toxicity
The toxicity of capreomycin is similar to that of streptomycin, although it is said that VIIIth nerve damage and allergy are less common and renal impairment more common. Toxicity also appears more likely in the elderly. In one report, 35% of 772 patients treated with capreomycin developed elevated blood urea nitrogen. Other reports suggest that proteinuria is common and that renal impairment may occur in up to 20–25% of patients treated with capreomcyin. There are also case reports of capreomycin causing magnesium and potassium wasting, hypocalcemia, a Bartter'slike syndrome, alkalosis, and secondary hyperaldosteronism via a direct renal tubular toxic effect. A case of fatal toxic nephritis possibly due to capreomycin has been reported.