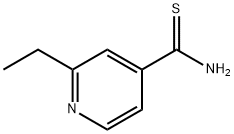
Mechanism of action of Ethionamide
Ethionamide (2-ethyl-4-pyridinecarbothioamide) is a derivative of isonicotinic acid that was first synthesized in France in 1956 and quickly shown to be an effective anti-anti-tuberculosis agent. It is closely related to isoniazid in both structure and function, but far more difficult for patients to tolerate, principally because of dose-related gastrointestinal side-effects. Subsequently an n-propyl derivative, prothionamide, was developed in an attempt to improve tolerability. Ethionamide and prothionamide frequently retain activity against isoniazid-resistant isolates of M. tuberculosis, hence their important reserve role in the management of drug-resistant tuberculosis. In clinical practice, ethionamide and prothionamide are generally regarded as equivalent. However there are subtle differences of uncertain clinical significance.
Mechanism of action
Mycobacteria have a similar inner lipid membrane and peptidoglycan cell wall to other bacteria but possess a second unique bilaminar outer component to their cell envelope, loosely analogous to the outer membrane of Gram-negative bacteria. However, its structure is asymmetric, with the inner component composed of molecules specific to the family Mycobacteriaceae: arabinogalactan and mycolic acids. Ethionamide interferes with the production of certain mycolic acids by first being activated to an ethionamide–NAD adduct, which then binds with InhA.
InhA is an NADH-dependent enoyl-ACP reductase of the type II fatty acid synthesis pathway. As its name suggests, InhA is also the target of isoniazid. Activation of ethionamide is mediated by a monooxygenase encoded by the gene ethA, but activation of isoniazid is mediated by a catalase–peroxidase encoded by different gene, katG. The binding of NAD–ethionamide/isoniazid adducts to InhA is competitively inhibited by intracellular NADP.
Another mechanism of resistance to ethionamide involves alterations in NADH dehydrogenase, encoded by nph. The mutations lead to increased levels of intracellular NADP and competition between NADP and activated drug. Resistance to isoniazid or ethionamide or both can usually be attributed to genetic mutations in katG, ethA (isolated ehtionamide resistance), or inhA/nph (isoniazid and ethionamide resistance). Recently, a new resistance mechanism for ethionamide was identified in the laboratory strain H37Rv that implicated mutations in mshA. This gene encodes a protein involved in mycothiol production. Although failure to produce mycothiol was non-lethal, these cells are ethionamide resistant. It is assumed that mycothiol production is somehow linked to the activation of ethionamide.
Bioavailability
Ethionamide is almost completely absorbed from the gastrointestinal tract, regardless of meals, and is not subject to significant first-pass metabolism. When given by suppository to 12 healthy volunteers the area under the serum concentration–time (AUC) curve was only 57.3% of that obtained when the dose was given orally. Protein binding is estimated at 30% but this drops significantly in malnourished patients.
Toxicity
Ethionamide has been described as the most difficult of the reserve agents for tuberculosis to tolerate. It is suggested that side-effects are related to serum level rather than route of administration as they also occur after systemic administration.
Hypoglycemia, hypothyroidism, acne, and other skin rashes have also been attributed to ethionamide. The management of patients with diabetes mellitus may become more difficult in those receiving ethionamide.
Lastest Price from Ethionamide manufacturers
US $0.00/kg2025-11-21
- CAS:
- 536-33-4
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 536-33-4
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 20 tons