Vanadium monocarbide:Structure,Uses,Preparation
Vanadium monocarbide is a tough and refractory ceramic material.
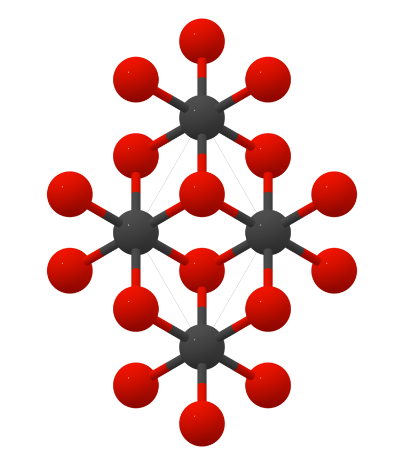
Crystal structure
Vanadium monocarbide has a hexagonal structure.
Uses
Vanadium monocarbide is a thermodynamic material used as an activation energy source to produce nitrides and carbides. Vanadium has a high melting point, which makes it ideal for applications where high temperatures are involved.
Preparation
To produce vanadium monocarbide, the metal must be heated to a temperature of approximately 2200 degrees Celsius. This material has been shown to have a particle size of less than 1 micrometer and can be activated by light or heat. The elemental composition of vanadium monocarbide is approximately 50% V and 50% C, with some impurities such as nitrogen atoms present.
Related compounds
Vanadium forms with carbon plenty of chemical compounds: vanadium monocarbide VC1–x having the broad homogeneity range with the highest C/V ratio being extremely far from the stoichiometry, several modifications of vanadium semicarbide:
α-V2+xC (Pbcn), β-V2±xC (P63/mmc) and β′-V2+xC (P(–3)m1, P(–3)1m, P312, Pnma, R(–3)m, P4/mmm, I41/amd, P3m1), some ordered and metastable phases: V5C3±x (I4/mcm), V3C2±x (R(–3)m, Immm, C2221, I4/mmm, C2/m, Immm, P(–3)m1), ζ-V4C3–x (or ξ-V3C2+x; R(–3)m, Pnma, P(–3)m1, C2/c, Pm(–3)m), V5C4±x (P(–1), C2/m, I4/m), V6C5±x (P3112, P31, P32, C2/m, C2/c, C2), V7C6±x (R(–3)) and V8C7±x (P4132, P4332), some subcarbides VxC (or more probably ordered V-C solid solutions) and metallocarbohedrene V8C12.