Crystal Structure, Applications and Synthesis Methods of Tungsten Silicide
With the extremely fast growth of technology and industry in the last decades has come the constant need for materials with improved characteristics and better performance under extreme conditions. In the metallurgical and the chemical industry, and in advanced technologies, the materials used are frequently subjected to extreme conditions such as exposure to chemical and mechanical stresses, and operation at high temperatures. Tungsten silicide, and in particular tungsten disilicide (WSi2) with melting point above 2160 °C and pentatungsten trisilicide (W5Si3) with melting point above 2320 °C, respectively, appear suitable for operation in extreme conditions[1]. This article will introduce the crystal structure and properties of tungsten silicide.
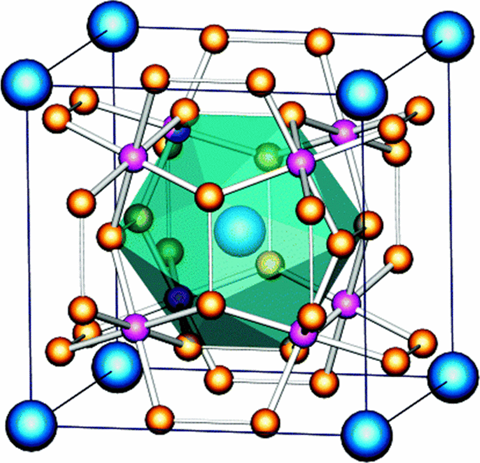
Crystal Structure
The figure above shows the crystal structure of tungsten silicide (W5Si3)[2].
Applications
WSi2 has low electrical resistivity and good thermal stability, and it has been investigated as a protective coating on W-based alloys because of its excellent oxidation resistance. Another area of application are integrated circuits, where one goal is to replace polycrystalline silicon with materials with lower resistivity. Thus, refractory metal silicides have been introduced as possible candidates for this application. Furthermore, W5Si3 coatings exhibit great abrasive and adhesive wear resistance. Tungsten silicides are also used as additives, where WSi2 and W5Si3 are included as suitable materials for mixing with e.g. WC, in order to overcome the problem of oxidation in extreme conditions, for applications in the metal and cutting tools industries[1].
Synthesis Methods
In the past, a variety of synthesis methods have been used for the production of tungsten silicide. Several different approaches can be distinguished: deposition methods for formation of thin films (by sputtering), by ion beam bombardment, or ion beam mixing with rapid thermal annealing, by low-pressure chemical vapor deposition, and by cold wall deposition (employing CVD). Kim et al. have discussed the transformation of WSi2 into W5Si3 at elevated temperatures, which has been experimentally confirmed by Kharatyan et al.
References
[1] Tungsten Disilicide (WSi2): Synthesis, Characterization, and Prediction of New Crystal Structures. doi: 10.1002/zaac.201700329
[2] W5Si3 (D8m) Structure: A3B5 tI32 140 ah bk-001. The AFLOW Library of Crystallographic Prototypes: Part 2, Comput. Mater. Sci. 161, S1 (2019).