Cobalt antimonide:Structure,Properties,Synthesis
The nanosized cobalt antimonide skutterudites are potential intermediate-temperature thermoelectric materials with further application in developing efficient thermoelectric modules.
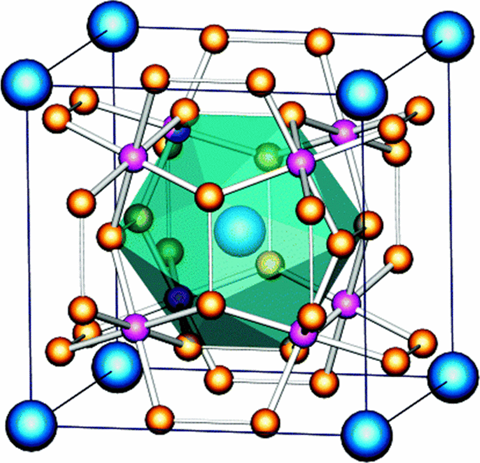
Crystal structure
Cobalt antimonide has a Cubic, Skutterudite Structure - Space Group Im3.
Properties
Cobalt antimonide is known for its attractive thermoelectric properties, i.e. good electrical conductivity and thermopower combined with relatively low thermal conductivity. Its applications in thermoelectric devices, such as power generators or coolers, are however limited due to the low thermal and chemical stability.
Both components of cobalt tri antimonide, cobalt, and antimony, exhibit a high affinity for oxygen. Several binary and ternary oxide phases can be formed at elevated temperatures and some of the oxidation products are volatile, which implies significant material losses. Numerous efforts have been undertaken to prevent the degradation of cobalt triantimonide in service conditions.
The best protection developed so far is provided by an aerogel coating, effective in the conditions of dynamic vacuum at temperatures not exceeding 450°C.
Synthesis
Cobalt antimonide nanoparticles, a binary skutterudite structure, are synthesized by following the solvothermal method using water as solvent. The solvothermal processed powders are annealed to remove the excess Sb to achieve single-phase CoSb3 nanoparticles and are examined by X-ray diffraction (XRD) indicating the formation of the cubic phase of CoSb3.
The structural analysis by selected area electron diffraction (SAED) and the elemental composition of 1:3 for Co and Sb using energy dispersive X-ray spectrophotometer (EDX) predicts the formation of high-purity crystalline CoSb3 nanoparticles. Morphology of the annealed CoSb3 powders observed using field emission scanning electron microscope (FESEM) and transmission electron microscopy (TEM) indicates particle size of 50-100 nm. The ultraviolet-visible (UV-Vis) absorption spectroscopy estimates an energy gap of 3 eV.


