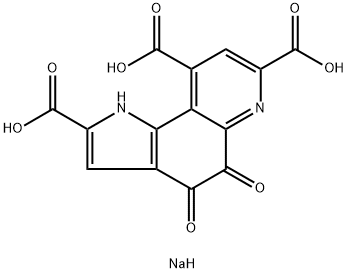
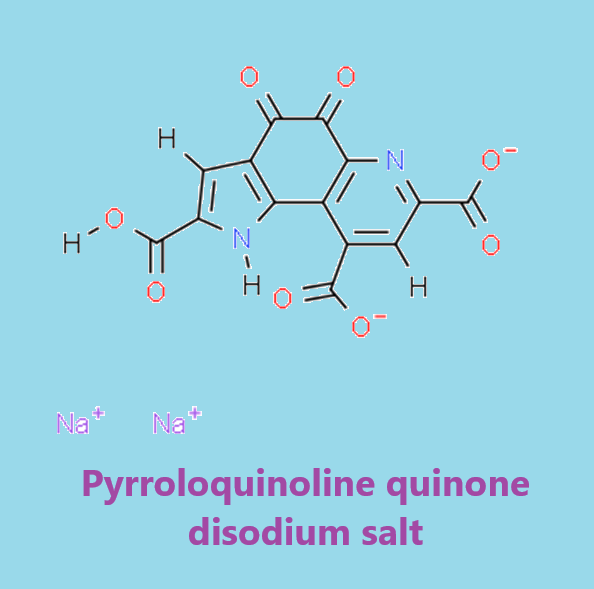
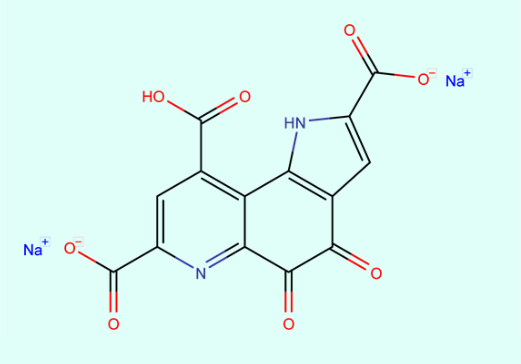
Pyrroloquinoline quinone disodium salt: Benefits and Safety
Pyrroloquinoline quinone disodium salt (PQQ) is a natural coenzyme in food that has highly efficient antioxidant activity and improves a variety of functions such as mitochondrial activation, growth, repair, and protection of neuronal cells by increasing the expression of nerve growth factor (NGF) and NGF receptor. It also inhibits protofibrils of amyloid β formation and aggregation. PQQ supplements are also widely used in daily life.

Benefits of Pyrroloquinoline quinone disodium salt
The benefits of Pyrroloquinoline quinone disodium salt include:
Improving of brain function
Brain function is important for a good quality of life. Pyrroloquinoline quinone disodium salt (PQQ) has been proven to improve brain function and cognition in older adults (above 45 years). In a double-blind, placebo-controlled study of adults aged 20 to 65 years who were given PQQ (20 mg per day) for 12 consecutive weeks, participants showed improvements in both general and verbal memory after 12 weeks. In young adults (20-40 years old), PQQ improved cognitive function (cognitive flexibility, processing speed, and executive speed) after 8 weeks. Only the elderly (41-65 years) showed improvements in complex and verbal memory after 12 weeks.
Improving dry skin
Pyrroloquinoline quinone disodium salt is a water-soluble quinone compound with strong antioxidant capacity. It is more powerful than vitamin C in fighting free radicals. Studies have shown that oral administration of PQQ improves skin condition in female subjects with dry skin and in mice with impaired skin barrier function. Oral administration of PQQ (0.0089%, w/w for 6 weeks) significantly reduced the number of mast cells in the dermis and CD3⁺ T cells in the epidermis. In human studies, oral PQQ (20 mg/day for 8 weeks) significantly inhibited the increase in transepidermal water loss (TEWL) in the forearm.
Improving mitochondrial dysfunction
Pyrroloquinoline quinone disodium salt promotes the maintenance of mitochondrial membrane potential, which is important for ATP production. Mitochondria are the energy centre of the cell and are closely associated with a variety of diseases. Studies have shown that PQQ helps make more mitochondria. One study tested PQQ supplements in men who performed aerobic exercise for 6 weeks, which increased mitochondria by affecting certain proteins during exercise. Another study showed that PQQ supplementation increased mitochondrial activity.
Inhibit LDL cholesterol levels
In a randomised, placebo-controlled, double-blind trial of 29 healthy Japanese adults (aged from 40 to 57 years), the results showed a marginally significant decrease in mean LDL-chol (from 136.1 to 127.0 mg/dL) in the PQQ group after 6 and 12 weeks of oral PQQ disodium salt (BioPQQ™) 20 mg /d treatment. In stratified analyses of the high ldl -cholesterol subgroup (baseline ldl -cholesterol level ≥140 mg/dL), the mean ldl -cholesterol level in the PQQ group declined significantly from the baseline value compared with the placebo group. Oral PQQ suppressed ldl -cholesterol levels.
Safety of Pyrroloquinoline quinone disodium salt
In a safety evaluation of the novel dietary Pyrroloquinoline quinone disodium salt (mnemoPQQ®). Acute toxicity studies of mnemoPQQ® in Wistar rats revealed that its LD50 was 1825- and 1410 mg/kg body weight (bw) in male and female rats, respectively, whereas its acute dermal LD50 was >2000 mg/kg bw. mnemoPQQ® was found to be nonirritant to the skin of rabbit in an acute dermal irritation/corrosion study, and classified mnemoPQQ® as a nonirritant to the eye of rabbit in an acute eye irritation/corrosion study. Ames bacterial reverse mutation assay and in vitro Mammalian cell gene mutation test exhibited its non-mutagenic potential. In mammalian in vivo erythrocyte micronucleus test, mnemoPQQ® was classified as non-clastogenic and non-mutagenic. A 90-day sub-chronic toxicity study, conducted at and up to the highest daily dose of 600 mg/kg body weight, revealed no evidence of systemic toxicity. All rats survived the treatment without any significant abnormal clinical signs and alterations in hematology, clinical chemistry, neurological evaluation, thyroid functions, reproductive hormone levels, sperm evaluations, vaginal cytology, endocrine functions, organ weight and gross and microscopic pathology findings. No observed adverse effect level (NOAEL) of mnemoPQQ® was found to be greater than 600 mg/kg body weight. These studies affirm that mnemoPQQ® has broad spectrum safety for human consumption.
A randomized, double‐blind, placebo‐controlled, parallel‐group clinical investigation (RCT) was conducted over a period of 12 weeks of supplementation of PQQ disodium salt (mnemoPQQ®; p.o.; 21.5 mg/day) in 64 healthy Japanese male and female volunteers (age 40 ‐<80 Y) to determine the safety and efficacy on improved cognitive function and performance. In another independent study, mnemoPQQ® exhibited broad spectrum safety in a battery of toxicological assessments. No mutagenic potentials were observed. The efficacy of mnemoPQQ® on cognitive performance (memory, attention, judgment, and cognitive flexibility) was examined using Cognitrax as the primary outcome (endpoint), and forgetfulness questionnaire (DECO: Deterioration Cognitive Observee) and Mini‐Mental State Examination‐Japanese (MMSE‐J) as the secondary outcomes (endpoints). A total of 58 subjects (Placebo group = 31; Age = 70.91 ± 3.06 Y, and mnemoPQQ® group = 27; Age = 72.10 ± 3.77 Y) completed the study over a period of 12 weeks. Significant improvements were observed on the Cognitrax’s cognitive function domain score on “composite memory”, “verbal memory”, “reaction time”, “complex attention”, “cognitive flexibility”, “executive function”, and “motor speed” in the mnemoPQQ® group as compared to the placebo group. The DECO index and the MMSE‐J score were also significantly improved in the mnemoPQQ® group. No adverse events were reported. This investigation demonstrates that supplementation of mnemoPQQ® is safe and efficacious in improving memory, attention, judgment, and cognitive function, in middle‐aged to elderly population.
In addition, PQQ disodium salt has no genotoxic activity in vivo.
References:
[1] Y. SHIOJIMA. Safety and Efficacy of a Novel Dietary Pyrroloquinoline Quinone Disodium Salt on Cognitive Functions in Healthy Volunteers: A Clinical Investigation[J]. FASEB Journal, 2022. DOI:10.1096/fasebj.2022.36.s1.r2889.
[2] TAMAKOSHI M, SUZUKI T, NISHIHARA E, et al. Pyrroloquinoline quinone disodium salt improves brain function in both younger and older adults†[J]. Food & Function, 2023. DOI:10.1039/D2FO01515C.
[3] MASAHIKO NAKANO. Effects of Orally Administered Pyrroloquinoline Quinone Disodium Salt on Dry Skin Conditions in Mice and Healthy Female Subjects.[J]. Journal of nutritional science and vitaminology, 2015. DOI:10.3177/jnsv.61.241.
[4] MASAHIKO NAKANO. Effects of Pyrroloquinoline Quinone Disodium Salt Intake on the Serum Cholesterol Levels of Healthy Japanese Adults.[J]. Journal of nutritional science and vitaminology, 2015. DOI:10.3177/jnsv.61.233.
[5] Y. SHIOJIMA. Safety assessment of a novel, dietary pyrroloquinoline quinone disodium salt (mnemoPQQ®)[J]. Toxicology Mechanisms and Methods, 2022. DOI:10.1080/15376516.2022.2076635.
Related articles And Qustion
See also
Lastest Price from Pyrroloquinoline quinone disodium salt manufacturers

US $1.00/KG2025-10-14
- CAS:
- 122628-50-7
- Min. Order:
- 25KG
- Purity:
- 99%
- Supply Ability:
- 20T

US $0.00-0.00/kg2025-08-19
- CAS:
- 122628-50-6
- Min. Order:
- 1kg
- Purity:
- ≥98%(HPLC)
- Supply Ability:
- 2tons