Uses of EGCG (Epicatechin-3-gallate) in Cosmetics
Overview
Green tea (Camellia sinensis) is a popular beverage with many purported health benefits including antiinflammatory,
chemopreventative, antioxidant, sunscreen effects, and more. The active ingredients modulating
those benefits in green tea are polyphenols called catechins: epicatechin-3-gallate, epigallocatechin,
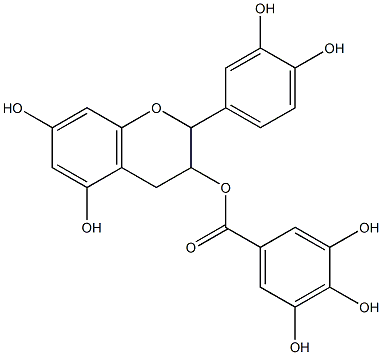
and epicatechin are three major catechins. Epicatechin-3-gallate, EGCG (Figure 4.1) is most abundant,
accounting from 50%–80% of the catechins in a cup of green tea. EGCG has been investigated for cosmetic
and medicinal uses; Table 4.1 lists several current products containing green tea extract.
Uses of EGCG in Cosmetics
Anti-Aging Uses
EGCG may have uses as an anti-aging or age-delaying agent. Dermal fibroblasts generate and maintain the collagen in the dermis and are important in wound healing. In vitro experiments using serially passaged human dermal fibroblasts in the presence and absence of 100 μM EGCG significantly prevent senescence of dermal fibroblasts. This effect appears to occur through p53 acetylation, although the importance for the role of p53 acetylation has been debated.3 This phenomenon also causes a change in cell morphology. However, reaching a 100 μM concentration of EGCG will be difficult to achieve through diet alone. A typical tea bag can contain anywhere from 1.5–3.3 g of green tea depending on the manufacturer. Consuming 3 g of tea dissolved in 500 mL of water a day leads to a plateau of 300 ng/mL of EGCG in the blood plasma of humans.
Hair Growth
Both in vitro and in vivo experiments were conducted on dermal papilla cells, which nourish and serve a function in hair follicle growth. The in vivo experiments involved applying up to 5 μM of EGCG in ethanol on the human occipital scalp for four consecutive days in two areas before tissue samples containing hair follicles were excised. Results showed a threefold increase in the expression of proteins involved in the increased proliferation of normal human epidermal keratinocytes. The in vitro experiments showed a dose-dependent increase in dermal papilla cell proliferation. Ex vivo experiments utilizing human hair follicle organ cultures treated for 10 days showed a 180% increase in hair follicle elongation.5 The biochemistry behind the actions of EGCG on hair growth have yet to be determined as well as its actions on different hair follicle cell types.
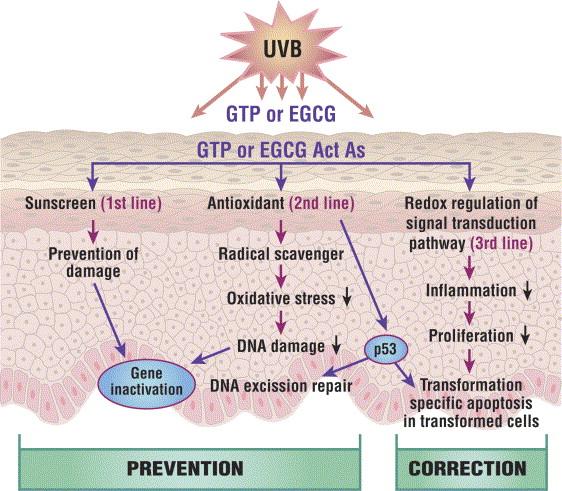
Sunscreen
Exposure to ultraviolet (UV) irradiation can lead to various skin cancers by mutating the DNA in epidermal cells, photoaging by degradation of collagen, immune suppression, and sunburns. UV radiation causes the release of reactive oxygen species (ROS) that damage DNA and lead to carcinogenesis. Certain polyphenols possess an antioxidant capacity to neutralize these free radicals.
Both UVA and UVB contribute to photoaging. The breakdown of collagen from UV exposure creates alterations in the extracellular matrix leading to wrinkling of skin. These alterations are mediated by matrix metalloproteinases, MMP, that are increasingly secreted by dermal fibroblasts in the presence of ROS.
Numerous studies have been conducted on the effectiveness of EGCG as a protective agent against UVA and UVB induced skin damage. Kim et al. performed an animal study on guinea pigs to study the preventative effects of EGCG against the UVB induced lipid peroxidation and erythema response. Hairless mice were used to determine the preventative effects of EGCG against UVA induced dermal collagenase activity. Both guinea pigs and mice were treated with a topical formulation consisting of the vehicle, 1% EGCG and 1% vitamin E before UV exposure. Results showed a 2.9-fold reduction in lipid peroxidation in the EGCG protected guinea pigs and a 1.6-fold difference between the erythema relative index of the control to the EGCG group. There were also significant decreases in collagenase activity and collagenase mRNA levels between the control and EGCG groups. EGCG and vitamin E appeared to perform similarily. Guinea pig skin protected by the EGCG formulation appeared to be less loose and rough compared to the control group. In vitro experiments support the results that EGCG inhibits the degradation of collagen and induction of collagenase caused by UVB exposure in dermal fibroblasts.
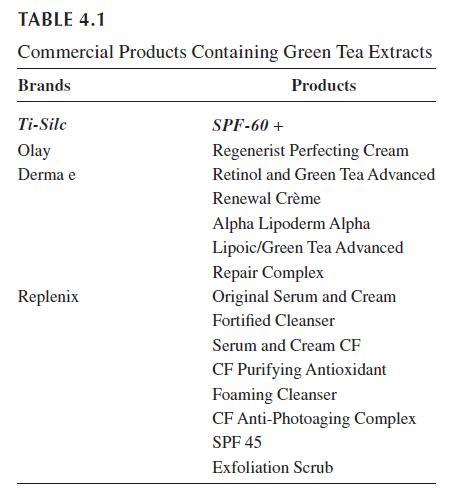
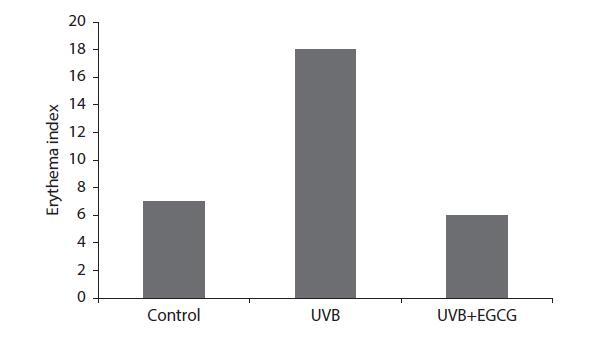
FIGURE 4.2 Topical application of EGCG reduces erythema to the applied buttocks site exposed to UVB in vivo human data. The control shin site was not exposed to UVB irradiation. 4 MED UVB dose was delivered to the sites and a chromometer was used to measure the intensity of the redness of the site. (Katiyar SK et al: Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem Photobiol. 1999. 69(2). Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.)
Studies focused on the protective effects of topical EGCG against UVA in albino rats revealed that applying the EGCG formulation 30 minutes before UVA exposure to the selected areas significantly reduced the number of sunburn cells in the epidermis of the rats. However, applying the formulation 30 minutes after exposure provided no benefits. Preventative experiments have been done on human subjects who were irradiated with four times the minimal erythema dosage (MED) on topically applied EGCG protected skin (Figure 4.2). Results showed a decrease in erythema and UVB induced infiltration of leukocytes, which are believed to be a major source of ROS.
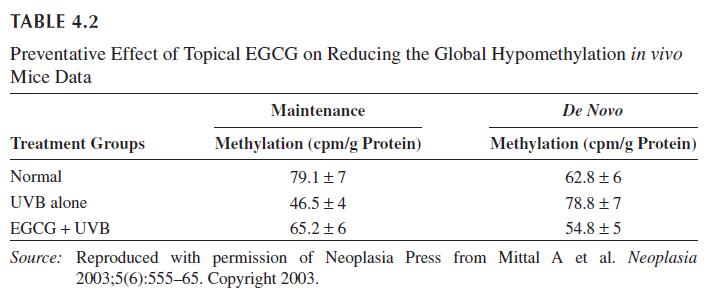
Oral formulations of EGCG have also been shown to be effective. Jeon et al. supplemented the diets of hairless rats with 1500 ppm of EGCG for eight weeks while subjecting the rats to UV radiation three times a week. EGCG supplemented mice had a mean MED value of 145 compared to the control group’s 99 (mJ/cm2). Furthermore, the EGCG mice had decreased trans epidermal water loss, TEWL, thereby suggesting better epidermal barrier functions. In vivo experiments support these results. Peripheral blood cells were drawn from adult human volunteers before and after drinking 540 mL of green tea. The cells were then exposed to UVA for 12 minutes. There was less DNA damage in eight of ten subjects who ingested green tea than those who had not. Altered DNA methylation silencing is a signal of cancer and Mittal et al. used monoclonal antibodies against 5-methyl cytosine and DNA methyltransferase in the long-term UV-irradiated skin in mice. Treatment with EGCG in a hydrophilic cream reduced global DNA hypomethylation, thereby reducing the incidence of skin carcinomas (Table 4.2). However, in another study oral doses of EGCG from 400 or 800 mg found that EGCG did not protect against UV induced erythema. This could suggest that topical formulations of EGCG may be better suited to reduce erythema. A study comparing oral and topical formulations of green tea extracts in 40 women with moderate photoaging found that there was no reduction in photoaging. This suggests that EGCG may perform better as a preventative product rather than repair-damaged tissue.
REFERENCES
1. Khan N et al. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate.
Cancer Res 2006;66(5):2500–5.
2. Han DW et al. Preventive effects of epigallocatechin-3-O-gallate against replicative senescence associated
with p53 acetylation in human dermal fibroblasts. Oxid Med Cell Longev 2012;2012:850684.
3. Prives C, Manley JL. Why is p53 acetylated? Cell 2001;107(7):815–8.
4. Yang CS et al. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by
human volunteers. Cancer Epidemiol Biomarkers Prev 1998;7(4):351–4.
5. Kwon OS et al. Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG).
Phytomedicine 2007;14(7–8):551–5.
6. Uitto J, Fazio MJ, Olsen DR. Molecular mechanisms of cutaneous aging. Age-associated connective tissue
alterations in the dermis. J Am Acad Dermatol 1989;21(3 Pt 2):614–22.
7. Cadet J et al. Effects of UV and visible radiation on DNA-final base damage. Biol Chem 1997;
378(11):1275–86.
8. Brenneisen P et al. Hydrogen peroxide (H2O2) increases the steady-state mRNA levels of collagenase/
MMP-1 in human dermal fibroblasts. Free Radic Biol Med 1997;22(3):515–24.
9. Wlaschek M et al. Singlet oxygen is an early intermediate in cytokine-dependent ultraviolet-A induction
of interstitial collagenase in human dermal fibroblasts in vitro. FEBS Lett 1997;413(2):239–42.
10. Kim J et al. Protective effects of (−)-epigallocatechin-3-gallate on UVA- and UVB-induced skin damage.
Skin Pharmacol Appl Skin Physiol 2001;14(1):11–9.