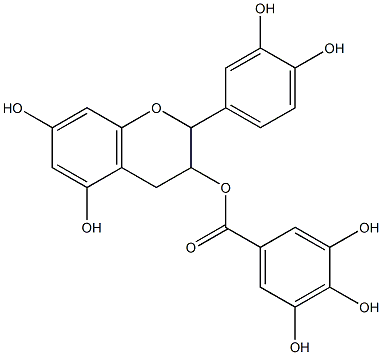
General Description of Epicatechin-3-gallate(EGCG)
Epicatechin gallate (ECG) is a flavan-3-ol, a type of flavonoid, present in green tea. It is also reported in buckwheat and in grape. The tea component epicatechin gallate is being researched because in vitro experiments showed it can reverse methicillin resistance in bacteria such as Staphylococcus aureus. Nevertheless, the compound is significantly degraded by steeping in boiling water, unlike related catechins.
Applications for EGCG in Skin Diseases
Keloids
Keloids are hyperproliferative fibrotic tissue deposition that occurs during skin healing. The majority of the material deposited is type III collagen that is later replaced by type I collagen. With the use of a keloid organ culture model, Syed et al. tested 100 μg/mL EGCG in vitro on keloid organ culture. EGCG decreased keloid volume by 40% on week 4 and increased apoptosis of keloid associated cells by the same amount. Collagen I and III were decreased at both the protein and mRNA levels, keloid mast cells were decreased by 98%, and EGCG induced epidermal shrinkage. EGCG also appears to inhibit keloid fibroblast proliferation and collagen production via inhibition of the STAT3 and the PI3K/AKT signaling pathways.
Wound Healing
EGCG has been demonstrated to be beneficial in the area of wound healing, although the mechanism is not fully understood. Cell culture studies with human keratinocytes reveal that EGCG enhances keratinocyte differentiation without promoting apoptosis. This suggests that EGCG may be useful in wound healing and skin diseases such as psoriasis that involve rapid turnover of undifferentiated keratinocytes. However, another study showed that EGCG alone did not alter human fibroblasts or keratinocyte proliferation. Combination of EGCG with alpha-lipoic acid was superior to EGCG alone in improving the rate of wound closure in mice. Aside from stimulating wound healing, EGCG is also effective against burn wound infections. Using nanoliposomes to increase the half-life and effectiveness of EGCG, the cationic EGCG loaded nanoliposomes were shown to decrease wound infection by Staphylococcus aureus. The killing rate of the EGCG reached 100%. The survival rate of the 10 mice treated by the cationic EGCG nanoliposomes was 100%. These results suggest that EGCG could be used as a topical formulation to enhance wound healing and decrease infection rates.
Psoriasis
Psoriasis, a disease of increased epidermal proliferation of keratinocytes, leads to a compromised barrier and inflammation due to an abnormal stratum corneum. Caspase 14 is associated with terminal keratinocyte differentiation, barrier formation, and with cornification and nuclear destruction of the keratinocyte cells but its expression is altered in human psoriatic tissue. Hsu et al. used both human epidermal keratinocyte cell cultures and flaky skin mice to determine if the use of a topical EGCG formulation can modulate the expression of the caspase 14 protein. The flaky skin mice bathed in 0.5% EGCG had more than twofold greater expression of the catalytically active form of the caspase 14 than the control mice had. The mice treated with EGCG also showed improved clinical and histological signs compared to that of the control mice.
Acne Vulgaris
Acne vulgaris is a complex chronic skin disorder that is prevalent in more than 85% of adolescents in the United States.26 Increased sebum production, inflammation, and Propionibacterium acnes (P. acnes) activity are three major processes that promote acne. Seb-1 human sebocytes treated with EGCG were found to have decreased lipid production (Figure 4.3). EGCG mitigated the inflammatory responses induced by exposure to P. acnes. These anti-inflammatory effects are supported in another study where SZ95 sebocytes were treated with EGCG and found to have decreased levels of inflammatory cytokines. 29 EGCG also inhibits growth of cultured P. acnes colonies. Furthermore, treatment with EGCG led to increased sebocyte apoptosis as well as decreased cellular growth in vitro. Topical application of EGCG has also been shown to reduce the size of sebaceous glands in rabbit auricles. Most significantly, EGCG was able to decrease both inflammatory and non-inflammatory lesion counts on humans receiving topical 1% or 5% EGCG solutions twice a day. These results suggest that EGCG could be an effective therapeutic treatment for acne vulgaris.
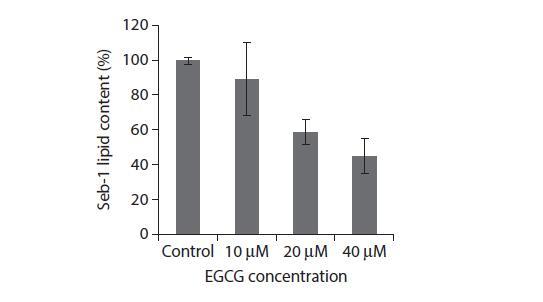
FIGURE 4.3 In vitro lipid content of Seb-1 sebocytes after 24 h treatment with EGCG. (Reprinted by permission from Macmillan Publishers Ltd. J Invest Dermatol. Yoon JY et al. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. 2013;133(2):429–40. Copyright 2013.)
Atopic Dermatitis
Atopic dermatitis, a chronic autoimmune disease that affects the skin, is characterized by allergic inflammation and local and systemic immune dysfunction leading to an imbalance of Th1 and Th2 cells. Macrophage migration inhibitory factor (MIF) is a crucial immunoregulatory cytokine in the pathogenesis of eczema. MIF is a pro-inflammatory cytokine that prolongs the inflammatory response. Considering that EGCG has an anti-inflammatory effect, mice were treated with Dermatophagoides pteronissinus extract (DPE) to induce eczema like lesions on the ears and treatment with topical EGCG was performed. After three weeks of treatment, ear thickness of the EGCG group was 41.6% less than that of the vehicle treated control and MIF was downregulated to be 1/3 less than the control. Mast cells contribute to the release of the cytokine thymic stromal lymphopoietin, TSLP, which plays a role in the progression of allergic diseases such as atopic dermatitis. In vitro studies have been conducted on human mast cell lines treated with EGCG and a dose dependent drop in TSLP was observed. As previously discussed, EGCG has antibacterial properties, which also make it useful in the treatment of eczema. Staphylococcus aureus is known to be an exacerbating factor in eczema and produces superantigen staphylococcal enterotoxin B, SEB. Treatment with EGCG on mice injected with SEB decreased the lethal toxicity of the toxin by 100%.
Toxicity and Pharmacokinetics
Toxicity studies suggest the safety of using high concentrations of EGCG. Studies determined that a 200 mg/kg oral dose of EGCG induced no toxicity in rats while increasing the dose by ten times was lethal. Rats tolerated up to 500 mg/kg/day of EGCG without toxicity. 500 mg/kg/day doses were also fed to dogs without any side effects; however, when the same concentration was fed to fasted dogs, it caused frequent diarrhea, occasional vomiting after feeding, toxic tubular necrosis, liver necrosis, hemolytic anemia, and moderate stomach erosion. Toxicity studies were also conducted on topical formulations that was a preparation of 93% EGCG in water at a dose of 2000 mg/kg and covered with a dressing. Minor irritation lasting five days was observed in rats and guinea pigs, but not rabbits. Dermal sensitization studies were conducted on guinea pigs; intradermal injections of 0.09% EGCG was the greatest tolerable dose. A grade 3 erythema was produced but no necrosis. Dermal exposure to EGCG of up to 50% did not evoke any reactions in the animals. Furthermore, EGCG was found to be an eye irritant in rabbits. Pharmacokinetic studies regarding the percutaneous absorption of 10% EGCG in a hydrophilic ointment were performed on mouse and human cadaver skin. Intradermal uptake in mice was rapid and plateaued quickly with an intradermal uptake of up to 19% while the intradermal uptake in human skin was only 0.9% and plateaued by 8 h. Transdermal penetration was only observed in mice. Stability of 10% EGCG in hydrophilic ointment stored for six months was also determined. At 37°C, 10% of the EGCG in the ointment was lost after two days, but the same formulation augmented with 0.1% butylated hydroxytoluene (BHT) had 90% EGCG remaining after 130 days under the same conditions.
There is no significant increase in the bioavailability of EGCG in human blood plasma from increasing the consumption of EGCG from 219 to 328 mg. A 200 mg dose of EGCG, which equates to approximately 3 g of green tea, maintains near maximum bioavailability and avoids toxicity. Presumably additional toxicological and continued pharmacological data will extend the voracity of the above observations.
REFERENCES
1. Bae JY et al. (−)Epigallocatechin gallate hampers collagen destruction and collagenase activation in
ultraviolet-B-irradiated human dermal fibroblasts: Involvement of mitogen-activated protein kinase.
Food Chem Toxicol 2008;46(4):1298–307.
2. Sevin A et al. Effects of polyphenols on skin damage due to ultraviolet A rays: An experimental study on
rats. J Eur Acad Dermatol Venereol 2007;21(5):650–6.
3. Katiyar SK et al. Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea reduces UVBinduced
inflammatory responses and infiltration of leukocytes in human skin. Photochem Photobiol
1999;69(2):148–53.
4. Jeon HY et al. Effects of oral epigallocatechin gallate supplementation on the minimal erythema dose and
UV-induced skin damage. Skin Pharmacol Physiol 2009;22(3):137–41.
5. Morley N et al. The green tea polyphenol (−)-epigallocatechin gallate and green tea can protect human
cellular DNA from ultraviolet and visible radiation-induced damage. Photodermatol Photoimmunol
Photomed 2005;21(1):15–22.
6. Mittal A et al. Exceptionally high protection of photocarcinogenesis by topical application of (−)-epigallocatechin-
3-gallate in hydrophilic cream in SKH-1 hairless mouse model: Relationship to inhibition of
UVB-induced global DNA hypomethylation. Neoplasia 2003;5(6):555–65.
7. Chow HH et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration
of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res 2003;9(9):3312–9.
8. Chiu AE et al. Double-blinded, placebo-controlled trial of green tea extracts in the clinical and histologic
appearance of photoaging skin. Dermatol Surg 2005;31(7 Pt 2):855–60; discussion 860.
9. Syed F et al. Ex vivo evaluation of antifibrotic compounds in skin scarring: EGCG and silencing of PAI-1
independently inhibit growth and induce keloid shrinkage. Lab Invest 2013;93(8):946–60.
10. Park G et al. Green tea polyphenol epigallocatechin-3-gallate suppresses collagen production and
proliferation in keloid fibroblasts via inhibition of the STAT3-signaling pathway. J Invest Dermatol
2008;128(10):2429–41.